Introduction
Epidemiological studies that demonstrate the adverse effects of increased levels of air pollutants on children's respiratory health (such as the incidence of acute conditions, chronic diseases exacerbation, lung function impairment or a prevalence of chronic ailments) are widely reported in the scientific literature1-6. As Schwela reported7, with long-term exposure to air pollution, children rather than adults exhibit increased respiratory symptoms such as chronic cough and reduced lung function. Air pollution itself, and also residential proximity to an industrial area8-12, waste site13 or US Environment Protection Authority (EPA)-identified pollution release sources14 are associated with impaired health of the respiratory tract in children.
The main suspected polluter in the study area - the region's industrial park (IP) situated in the Negev, southern Israel - is one of the largest chemical manufacturing sites in Israel; it consists of 17 plants and an all-country toxic industrial waste dump with an incinerator. Because local residents had complained about odours coming from the IP and blamed its emissions for worsening health conditions over a long period, regional health authorities requested that epidemiologists from the Ben-Gurion University of the Negev investigate possible negative effects.
In previous ecological studies, an association was found between residential proximity to the IP and adverse health effects in the Negev Bedouin population15-17. Thus, the objective of the present study was to investigate a hypothesis that linked IP proximity with life prevalence (LP) of upper respiratory tract chronic diseases (URTCD) and asthma in children aged 0-14 years living in small agricultural communities (kibbutzim) in the Negev area. The study investigated whether indirect indicators of exposure to the IP emissions (a child's kibbutz distance from the IP; the kibbutz's location in relation to the dominant wind direction from the IP; or the child's mother's complaints about odour related to the IP) or other variables (demographic characteristics; the child's birth history; and various parental and local environment factors) were related to the child's chronic respiratory morbidity.
Methods
This cross-sectional study was conducted during 2002. All medical diagnoses relevant to chronic respiratory conditions were collected simultaneously in all locations during the summer of 2002.
Study area and target population
Kibbutzim (plural of 'kibbutz', Hebrew for communal settlement) are unique rural communities, dedicated to mutual aid and social justice18. Their socioeconomic system is based on the principle of joint ownership of property, and equality and cooperation in production, consumption and education. Most kibbutzim are laid out according to a similar plan. The residential area encompasses carefully tended members' homes and gardens, children's houses and playgrounds for every age group, and communal facilities such as a dining hall, medical clinic, auditorium, library, swimming pool, tennis court, laundry, and grocery area etc. Adjacent to the living quarters are sheds for dairy cattle and modern chicken coops, as well as one or more industrial plants. Agricultural fields, orchards and fishponds are located around the perimeter, a short tractor ride from the centre.
The study kibbutzim were comparable regarding types of agriculture (ie field, orchard, landscape and livestock), small industries (plastic and metalwork), stable population, and socioeconomic characteristics (including access to the health service in local kibbutzim clinics).
Source of the region's environmental pollution
The IP was established and erected during the 1970s, and now it hosts 17 industrial facilities (including chemical, pharmacochemical and heavy industry). For the past 25 years, the IP has also housed the national industrial hazardous waste disposal site. Each year 35 000 tonnes of toxic wastes (mainly industrial sludge and petrochemical waste, agents containing heavy metals, pesticides and solvents) are transferred to the site15-17. In 1997, an incinerator for toxic organic waste was constructed, which has burned more than 20 000 tonnes of waste each year since15-17.
The list of IP emissions from its industrial facilities and evaporation pools includes a variety of aliphatic, aromatic, and polycyclic hydrocarbons as well as several dozen inorganic substances (including heavy metals)15-17. At the IP site, the air is monitored every 30 min for concentrations of inorganic compounds (H2S, Cl, Br, HBr, and HCl) and every 24 hours for concentrations of 61 organic compounds (such as benzene and its halogenated, methylated, and ethylated derivates, benzaldehyde, hydroxy benzaldehyde, benzonitrile, cyclohexene, dimethylamine, naphthalene, tetrahydronaphthalene, toluene, xylene, bromoxylene, acetone, terpinene, methane, ethane, and ethene halogenated compounds, carbon disulfide and tetrachloride, chloroform, dimethyl sulfides, ethyl acetate, ethylene bromide, isopropanol, methylene chloride, methyl-ethyl-ketone, methyl-iso-butyl-ketone, methyl-phenyl-ketone, phosphorodithioic acid trimethyl ester, tetrachloropyridine, and acetic acid)15-17. The IP authorities report periodically to the Ministry of Health, and most of the measurements (conducted by the IP Department of Environmental Protection) reveal low concentrations of pollutants at the IP site, up to hundreds of times below the limit level for occupational exposure15-17.
However, even if the proportions of occupational threshold level values (TLV) related to the measurements' results reflect 'safety coefficients' for populated places, the additive (synergetic) health effects of the combination of many pollutants (still under the TLV) should be taken into account. Although a large number of pollutants are being measured, it is possible that other toxic pollutants are emitted without proper monitoring. The region's semi-arid climate, characterized by frequent temperature inversions and low annual precipitation (15 to 300 mm), also reduces the air's 'self-cleaning'15-17.
Exposure indicators
Due to the lack of regular direct measurements of the ambient air pollution at the kibbutzim's sites, it was necessary to use the following indirect measurements of exposure: (i) a child's kibbutz distance from the IP; (ii) the dominant wind direction from the IP towards the child's kibbutz; and (iii) the child's mother's complaints about odour related to the IP.
The kibbutzim of interest happened to be located in two geographical clusters. The first cluster consists of 3 kibbutzim situated 13, 14 and 16 km from the IP (the average distance between them was 1.5 km; their average distance from the IP was 14.3 km). The second cluster consists of 4 kibbutzim situated 24, 28, 29 and 33 km from the IP (the average distance between them was 3 km; the average distance from the IP was 28.5 km). The proportion of the average distance between them to the average distance from the IP was similarly small at (1.5/14.3 =) 0.10 and (3/28.5 =) 0.11 for the first and second clusters, respectively. This means that the individual distance from the IP of any given kibbutz can be replaced (with a good accuracy) by the average amount of space between the IP and the cluster to which the given kibbutz belongs. These calculations demonstrate the irrelevance of the continuous distance variable in the present case. Thus, instead the study used the dichotomized distance variable containing two categories: (i) proximal (less than 20 km from the IP); and (ii) distal (greater than 20 km from the IP). The 20 km cut-off point was chosen because most odour complaints within a 20 km radius from the IP were related to the IP emissions (pers comm, T Galin [head of the Department of Environmental Protection, IP], 2005). Consequently, the proximal sector included the kibbutzim of the first geographical cluster (ie located 12, 14, and 16 km from the IP), and the distal sector included the kibbutzim of the second geographical cluster (ie those situated 24, 29, 30, and 33 km from the IP) (Fig1).
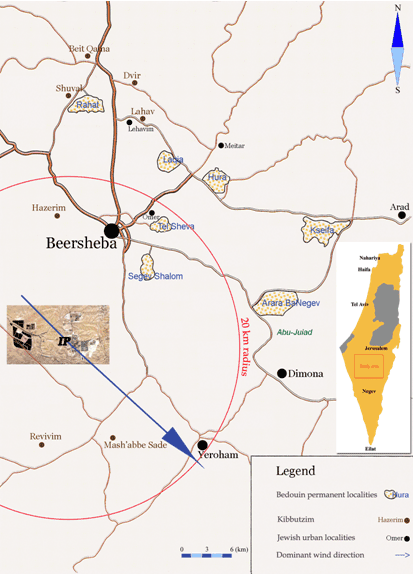
Figure 1: Map showing the industrial park and kibbutzim at Negev, Israel.
Among the 7 kibbutzim in the study area, all but one lay within the same dominant wind direction, which makes the continuous wind-direction variable extraneous as well. In its place the binary variable 'wind direction' was used, which indicated presence (yes/no) of the dominant wind direction from the IP towards a child's kibbutz. This indicator further divided the kibbutzim of the proximal sector into two subgroups: one kibbutz that was situated within the dominant wind direction, and two others outside it.
Both exposure indicators mentioned (the dichotomized distance from the IP and the indicator of the dominant wind direction), which can be called the 'objective' measures of exposure, are insufficient for describing the spread of air pollution (such a distribution would be complete only with the use of a full bench of real-time detectors scattered over the study area). Therefore, it was necessary to include a 'subjective' measure of exposure - the yes/no variable indicating maternal complaints about odour, which contributed to providing a relatively complete set of exposure variables.
Regarding the concept of maternal complaints about odour, the odour related to the IP is unique and quite different from other irritant environmental odours; it was complaints about this specific odour that generated the present study. So serious and 'objective' was this 'subjective' variable that a special phone-service centre was established in the area to deal with residents' complaints of odour. Each time a complaint was registered, air sampling and chemical analysis were performed at the scene of the complaint (as well as measurements of meteorological parameters). Therefore, one of the purposes of the present research was to discover it this variable was associated with a child's health status.
Study population
The study population included children aged 0-14 years born in the kibbutzim. Informed consent was provided by: (i) the parents of the 333 children (71% response rate) who lived in the proximal kibbutzim, which includied 97 individuals from one kibbutz within the dominant wind direction; and (ii) the parents of the 217 children (70%) who lived in the distal kibbutzim. The total study population was 550 children.
Data sources
The data about URTCD, which included chronic rhinitis, pharyngitis, nasopharyngitis, sinusitis, tonsillitis (ICD9 ##472-474) and asthma (ICD9 #493) diagnosed by a medical doctor, were collected from kibbutz clinic records.
A child's respiratory health was measured by the LP of URTCD and asthma, where LP was defined as prevalence (%) during the first 0-14 years of life.
The following elements were included in the interviewer-administered questionnaire for the child's parents:
- Demography: the child's birth date, gender, mother's marital status, parental origin and education, and number of siblings.
- The child's birth history (pregnancy, delivery) and breast feeding duration.
- The child's active smoking.
- The child's parental respiratory health.
- Environmental factors: parental smoking and occupational hazardous exposure, domestic use of pesticides, domestic animals, outdoor odour related to IP emissions.
Statistical analysis
For univariate analysis, the distributions of LP of URTCD and asthma were compared (using Pearson's χ2) for values of the following (categorical) variables: demography, child's birth history, environmental exposure and parental respiratory health. In addition the following variables were tested (with t-tests): gestational age, birth weight, breastfeeding duration, child's age, and total number of children in family. These had the same mean within the two groups of children (with and without URTCD or asthma).
Multivariate analysis was performed using logistic regression models of the LP adjusted for clustering on the child's family identification number. To estimate odds ratio (OR) within 95% confidence interval (CI) of association between the disease LP and independent variables, those study variables that were significant at the level p <0.10 in the univariate analysis were fitted with multivariate logistic regression models. All statistical analyses were performed using Stata Statistical Software v9-10 (StataCorp LP; College Station, TX, USA).
The study proposal and text of the informed consent forms were approved by the Helsinki Committee of the Soroka University Medical Centre.
Results
The results will be presented in the following structure. First, the data for LP of URTCD, then the results for the entire study area, followed by data obtained only for the proximal sector, and the results of multivariate logistic regression models to conclude. Second, the data for asthma LP is placed in analogous order. The results presented include all the analyzed variables of the study, with the exception of the child's active smoking, for which there was no one positive answer.
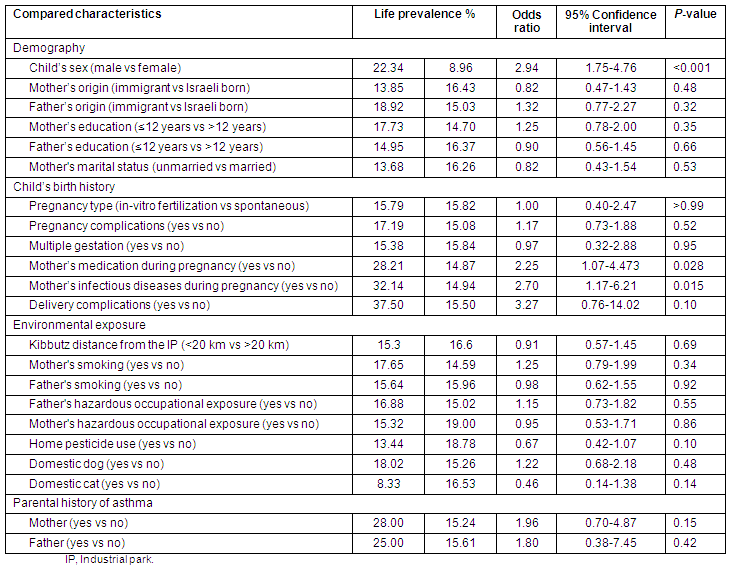
Life prevalence of upper respiratory tract chronic diseases among the children living in 7 kibbutzim
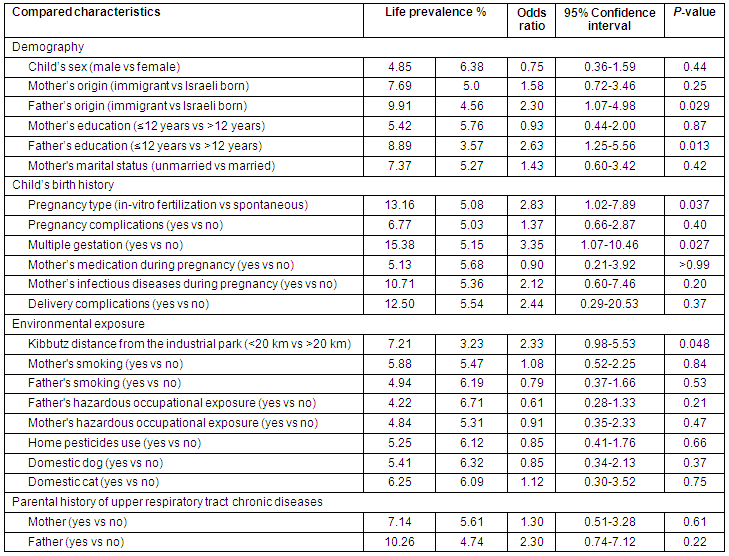
The high LP of URTCD (Table 1) was related to the following variables:
- A child's kibbutz is in the proximal sector
- The child's father is an immigrant
- The child's father's education is up to 12 years
- The child's mother had a multiple gestation pregnancy
- The child's mother had an in vitro fertilization (IVF) pregnancy.
Other not statistically significant variables were found that affected LP (Table 1).
Table 1: Life prevalence of upper respiratory tract chronic diseases in 550 children of 7 kibbutzim
Children with and without URTCD diagnosis were not statistically different in terms of the following variables:
- The child's age (years) (8.10 ± 3.68 vs 7.88 ± 4.05, p = 0.77)
- The child's number of siblings (3.29 ± 0.78 vs 3.15 ± 1.14, p = 0.34)
- The child's gestational age (weeks) (38.23 ± 7.27 vs 37.10 ± 8.90, p = 0.49)
- The child's birth weight (g) (3326 ± 529.31 vs 3217 ± 570.19, p = 0.27)
- The child's breastfeeding period (months) (3.42 ± 1.68 vs 3.79 ± 2.93, p = 0.31).
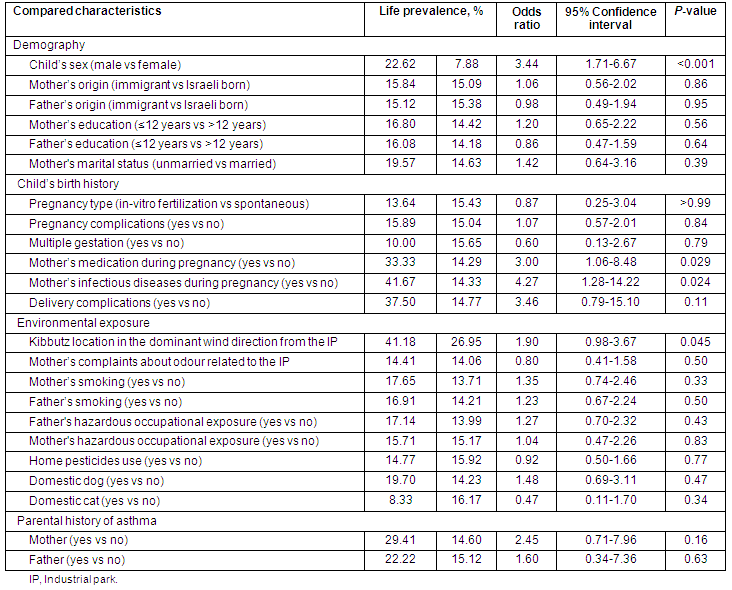
Life prevalence of upper respiratory tract chronic diseases among the children living in 3 kibbutzim proximal to the industrial park
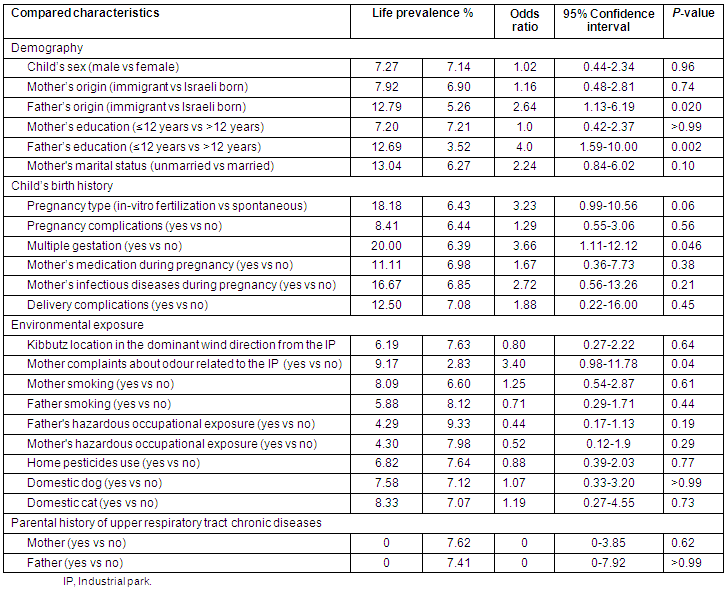
As shown (Table 2), the increased LP of URTCD was associated with:
- The child's mother's complaints about IP-related odour
- The child's father bring an immigrant
- The child's father's education being up to 12 years
- The child's mother had a multiple gestation pregnancy
- The child's mother had an in vitro fertilization (IVF) pregnancy.
Other non-statistically significant variables were found that affected LP (Table 2).
Table 2: Life prevalence of upper respiratory tract chronic diseases in 333 children of 3 kibbutzim proximal to the industrial park
Children with and without URTCD diagnosis were not statistically different in terms of the following variables:
- The child's age (years) (8.08 ± 3.89 vs 7.89 ± 4.02, p = 0.82)
- The child's number of siblings (3.33 ± 0.82 vs 3.20 ± 1.20, p = 0.46)
- The child's gestational age (weeks) (37.79 ± 8.23 vs 37.0 ± 9.17, p = 0.68)
- The child's birth weight (g) (3240 ± 612.70 vs 3208 ± 572.75, p = 0.79)
- The child's breastfeeding period (months) (3.47 ± 1.78 vs 3.79 ± 2.46, p = 0.60).
Multivariate logistic regression models of life prevalence of upper respiratory tract chronic diseases
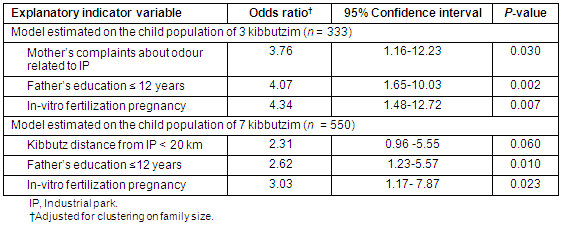
The final model of LP of URTCD estimated on the population of children of 3 kibbutzim (n = 333) contained only three explanatory (binary) variables: (i) mother's complaints about IP odour; (ii) father's education is less than or equal to 12 years; and (iii) mother's IVF pregnancy (Table 3). However, the final model estimated on the population of children of 7 kibbutzim (n = 550) also contained only three explanatory binary variables: (i) a child's kibbutz is proximal; (ii) father's education is less than or equal to 12 years; and (iii) the mother's IVF pregnancy.
Table 3: Final logistic regression models of life prevalence of upper respiratory tract chronic diseases
Asthma life prevalence among the children living in 7 kibbutzim
As in Table 4, the increased asthma LP in children of the 7 kibbutzim was related to:
- The child's male gender
- The child's mother's medication during pregnancy
- The child's mother's infectious diseases during pregnancy.
Regarding the distance from the IP and the other child's characteristics, the values for asthma LP are similar (Table 4).
Table 4: Asthma life prevalence in 550 children of 7 kibbutzim
Children with and without asthma were different when comparing by months of breastfeeding (3.03 ± 2.68 vs 3.91 ± 2.29, p = 0.01) and the average number of children in family (3.36 ± 0.12 vs 3.12 ± 0.05, p = 0.067). No difference was found for the child's age in years (8.01 ± 4.03 vs 7.87 ± 4.03, p = 0.76), the child's gestational age in weeks (37.55 ± 7.34 vs 37.09 ± 9.07, p = 0.61) and the child's birth weight in grams (3160 ± 527 vs 3235 ± 532, p = 0.23).
Asthma life prevalence among the children living in the proximal kibbutzim
Table 5 shows that high asthma LP was associated with:
- The child's kibbutz location within the dominant wind direction from the IP
- The child's male gender
- The child's mother's medication during pregnancy
- The child's mother's infectious diseases during pregnancy.
The difference between asthma LP for other characteristics of the population was not significant (Table 5).
Table 5: Asthma life prevalence in 333 children of 3 kibbutzim proximal to the industrial park
Children with and without asthma were found to be similar according to:
- The child's age (years) (8.63 ± 3.59 vs 7.77 ± 4.07, p = 0.16)
- The child's number of siblings (3.43 ± 0.17 vs 3.17 ± 0.07, p = 0.139)
- The child's gestational age (weeks) (38.06 ± 5.75 vs 36.87 ± 9.58, p = 0.23)
- The child's birth weight (g) (3135 ± 533 vs 3224 ± 582, p = 0.31)
- The child's breastfeeding period (months) (3.31 ± 2.50 vs 3.85 ± 2.41, p = 0.15).
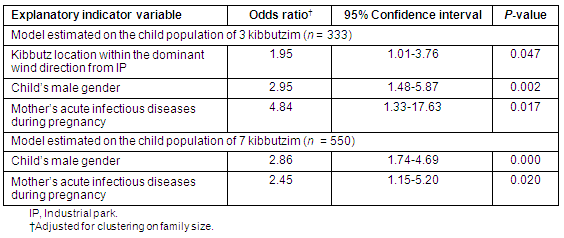
Multivariate logistic regression models of asthma life prevalence
The final model of asthma LP estimated on the population of children of 3 kibbutzim (n = 333) contained only three explanatory binary variables: (i) a child's kibbutz location within the dominant wind direction from the IP; (ii) the child's male gender; (iii) and the child's mother's acute infectious diseases during pregnancy (Table 6). The final model estimated on the population of children of 7 kibbutzim (n = 550) included only two explanatory binary variables: (i) the child's male gender; and (ii) the child's mother's acute infectious diseases during pregnancy (Table 6).
Table 6: Final logistic regression models of asthma life prevalence
Discussion
This study is the first of its kind on respiratory morbidity among children residing in kibbutzim in Negev located close to the regional industrial complex.
Chronic diseases of upper respiratory tract
For children residing in the proximal kibbutzim, a significant association was observed between the increased LP of URTCD in a child and his/her mother's complaints about odour from the IP. Comparing these children with those from the distal kibbutzim, a high LP of URTCD was observed for the former (at borderline statistical insignificance p = 0.06). This conclusion is biologically logical, given that the upper airway is the first line of defence against inhaled pollutants. A similar trend (health outcomes worsening in relation to the distance from of the IP) was been observed in the Bedouin population living in the same study area15-17.
Most studies of child respiratory epidemiology address disorders of the lower airways because it is there that more serious health effects are caused by pollutants. Similar information on URTCD is scarce. Some investigations considered residence in an industrially polluted area as a measure of potential exposure to emissions. The probability of rhinitis was found to be threefold greater (95% CI = 1.1-7.1) for children in Sri Lanka in an industrial area than that of same aged residents of a non-industrialized area12. Increased prevalence of sore throat and blocked nose were shown (after adjustment for confounders) among children in an area near cement works in small rural town in east Lancashire, compared with those residing far away from the plant area8. The authors assumed the symptoms were a good indicator of long-term exposure to air pollution. Yang and co-authors10 reported that schoolchildren living in a petrochemically polluted area in Taiwan had significantly more upper respiratory tract symptoms than those in a zone distant from those industrial facilities. High incidence of upper respiratory airway diseases was observed in children of 5-15 years living in greatly polluted regions of Poland, compared with those from less polluted areas19. In Germany, among 9-11 year-old children, upper respiratory tract symptoms were most prevalent in areas heavily polluted by industrial emissions20.
It can be assumed that exposure to IP emissions may cause some decrease in immune function, which leads to the development of chronic disease of the upper respiratory tract. Researchers associate immune system disorders with residence in air-polluted areas. For example, among residents of Aberdeen (North Carolina) near a 'Superfund' site (contaminated with organochlorine pesticides, volatile organic compounds and metals), decreased mitogen-induced lymphoproliferative activity was found21. Foltinova and co-authors22 reported on a case of tracheal damage due to exposure to various aerosols, that functional morphology was altered. In the authors' opinion, that finding assists understanding of the damaging mechanisms on mucociliary clearance induced by living in heavily polluted areas.
The examination of the nasal mucosa of 100 children aged 6-7 years living in different polluted areas shows that a child's upper airway epithelium had a lower purifying capacity, especially in those residing in the higher polluted area23. In a recent review24, the effects are presented of environmental exposures to volatile organic compounds, metals, and pesticides on Th1/Th2 cytokine profiles in children, and the association of Th1/Th2 profiles with adverse health outcomes (such as children's respiratory diseases). This immune response may be related to pollutant irritation ability or the impact of continuous odorous exposure. The potential for volatile chemicals to elicit chemosensory irritation in the upper respiratory tract is well recognized25. Odoriferous chemical vapour was found responsible for respiratory illness in the residential environment of schoolchildren in Taipei26.
On the role of the father's education in a child's respiratory health, the results of the Pollution and the Young (PATY) study indicate an association between low parental education and increased prevalence of wheeze and nocturnal dry cough27. In this study, parental education was used as an indicator of socioeconomic status. For kibbutzim population, such a definition of socioeconomic status is not applicable, and so the present study did not describe socioeconomic status. Indeed, the specific characteristic of the population group analyzed (ie kibbutzim members living a communal life style) makes the use of individual socioeconomic status irrelevant. Likewise, collecting individual information about alcohol intake and diet among kibbutzim residents was also unnecessary. The contribution of a father's lower education in increased LP of URTCD could be explained by a lower level of family knowledge about health protection.
The most unexpected finding in the present study was the association between the increased LP of URTCD and the child's mother's IVF. Similarly, Koivurova and co-authors28 in a cohort study (based on the general population in northern Finland) demonstrated that the cumulative incidence of respiratory diseases was significantly higher among IVF children of up to 3 years of age (OR = 3.5; 95% CI = 1.9-6.5). It is well recognized that multiple gestations follow IVF29, and in the present study multiple gestations had a strong association with LP of URTCD.
Asthma
A significant association was found between increased asthma LP and the child's residence in the kibbutz proximal from the IP located within the dominant wind direction from the IP. However, air pollution is usually considered more a factor in asthma exacerbation than its cause30,31. Nevertheless, in a petrochemical polluted area in Taiwan, high asthma prevalence was reported among the schoolchildren10. Asthma was also observed to be more common in the industrial area of Ahmedabad City, India32. The standardized incidence ratio for asthma was found significantly increased among inhabitants of Helsinki blockhouses built in on a former dump area containing industrial and household wastes33.
Taking into account the IP emission components described above under the heading 'Source of the region's environmental pollution', the information on respiratory epidemiology in relation to organic air pollutants was examined. The results of a cross-sectional study in Kanawha County in West Virginia (one of the largest chemical manufacturing centres in the United States) showed that children in the third to fifth grades enrolled in local valley schools had higher rates of diagnosed asthma and chronic respiratory symptoms than children who were enrolled in schools outside the valley34. This difference was explained by dissimilarity in industry-related air pollution measured at each school; the incidence of respiratory symptoms was positively associated with concentrations of volatile organic compounds specific to the industrial process.
Concerning the observed values of asthma LP, in the Israel national survey of 13-14 year-old schoolchildren, residence in a rural area was found to be a risk factor for asthma morbidity35. In addition, a high prevalence of asthma (13.7%) was observed in 8-17 year-old Jewish children in one Israeli town36.
The finding of a high proportion of asthma LP among boys is reflected in the literature37,38. As for the observed asthma LP association with a child's mother's acute infectious diseases during pregnancy, there is some controversy. While some studies suggest that infection in pregnancy can be a factor in increasing the risk of allergic disorders among born children39,40, another study indicates this factor might be protective of atopic disease41.
Parental smoking and history of asthma (in spite of being known for their contribution to child respiratory problems) were found not to be associated with observed health outcomes.
Strength and limitation of the study
Because direct monitoring of air pollution in the kibbutzim was not available, it was necessary to use an indirect measure of exposure to IP emissions. Using the three indicators instead: (i) distance (less than 20 km/ more than 20 km) of the child's kibbutz from the IP; (ii) presence (yes/no) of the dominant wind direction from the IP toward the child's kibbutz; and (iii) child's mother's odour complaints (yes/no) related to the IP, allowed some reduction of the risk of misclassification.
Using the presence of the dominant wind direction as an additional exposure indicator coincides with opinion expressed elsewhere42. These authors claimed that using circles as the only means of identifying the exposed population leads to the dilution of the potential health effect and, therefore, one of the best approaches is to use local knowledge about wind directions to identify the population likely to be at risk.
The present study results show the association of asthma and URTCD with different exposure indicators: the kibbutz location within the dominant wind direction from the IP was associated with asthma, but the residential distance from the IP and maternal complaints about odour were linked to URTCD. This difference is not considered contradictory. It is known that the pathogenesis of chronic inflammatory and allergic respiratory ailments is different, which may be attributed to immune system features and the intensity of the chemical impact of the environment. It can only be assumed that for asthma onset, the chemical 'press' would be more intensive - and this is what was observed for the proximal kibbutz location within the dominant wind direction.
Because of the equivalent, high percentage response in compared kibbutzim, selection bias is unlikely. And medical diagnoses from the kibbutz clinic records secure reduction of outcome bias. This is especially important for comparison of outcomes for the levels of such exposure indicators as maternal complaints about the IP-related odour. In this way the possibility of a relationship between adverse effect and exposure self-perception is avoided. While it is theoretically difficult to rule out a potential bias related to maternal odour complaints, if such a bias exists it would not be systematic. In reality, in all 3 kibbutzim located in the exposure zone (less than 20 km from the IP), this variable was insignificant to asthma incidences.
To increase association strength for the exposure indicators, adjustment for variables with confounding and modifying potential was not performed (in accordance with the literature). However, it should be noted that such factors as socioeconomic status, lifestyle, local detail environmental exposure, food consumption and medical care were removed, because the kibbutzim in this study were very similar in these variables. The associations found are biologically sound and consistent with other epidemiological studies.
Conclusions
Among Negev kibbutzim children, associations were found of increased LP of URTCD and asthma with indirect indicators of exposure to the IP; specifically, dominant wind direction was associated with asthma LP, and the distance and mother's complaints about odour were associated with LP of URTCD.
It is hoped that the results of the present study, combined with previously reported findings in the Negev Bedouin population15-17, will advance local health policy sufficiently to improve environmental conditions in the Negev.
Acknowledgments
The authors express their acknowledgment of the kibbutz nurses Riva Israelovich, Sarah Kassel, Orly Zilkenstein, Daphna Elyakim, Smadar Dagan, Anat Cohen, Ophra Feldman and Batia Weigel for their assistance and cooperation with the study team in retrieving the data from medical records. Mrs Tally Lahav's master of medicine thesis formed part of this study. The study was partially supported by the Israeli Ministry of Health, Contract # 89838; this funding covered the cost of data collection.
References
1. Bates DV. The effects of air pollution on children. Environmental Health Perspectives 1995; 103(Suppl6): 49-53.
2. Raizenne M, Dales R, Burnett R. Air pollution exposures and children's health. Canadian Journal of Public Health 1998; 89(Suppl1): S43-8, S47-53. (Review)
3. Romieu I, Samet JM, Smith KR, Bruce N. Outdoor air pollution and acute respiratory infections among children in developing countries. Journal of Occupational and Environmental Medicine 2002; 44(7): 640-649.
4. Schwartz J. Air pollution and children's health. Pediatrics 2004; 113(Suppl4): 1037-1043. (Review)
5. Ward DJ, Ayres JG. Particulate air pollution and panel studies in children: a systematic review. Occupational and Environmental Medicine 2004; 61(4): e13.
6. Bateson TF, Schwartz J. Children's response to air pollutants. Journal of Toxicology and Environmental Health 2008; 71(3): 238-243.
7. Schwela D. Air pollution and health in urban areas. Reviews on Environmental Health 2000; 15(1-2): 13-42.
8. Ginns SE, Gatrell AC. Respiratory health effects of industrial air pollution: a study in east Lancashire, UK. Journal of Epidemiology and Community Health 1996; 50(6): 631-635.
9. Gomzi M, Sarić M. Respiratory impairment among children living in the vicinity of a fertilizer plant. International Archives of Occupational and Environmental Health 1997; 70(5): 314-320.
10. Yang CY, Wang JD, Chan CC, Hwang JS, Chen PC. Respiratory symptoms of primary school children living in a petrochemical polluted area in Taiwan. Pediatric Pulmonology 1998; 25(5): 299-303.
11. Pilotto LS, Smith BJ, Nitschke M, Ruffin RE, Mitchell R. Industry, air quality, cigarette smoke and rates of respiratory illness in Port Adelaide. Australian and New Zealand Journal of Public Health 1999; 23(6): 657-660.
12. Premaratna R, Pathmeswaran A, Chandrasekara B, Dissanayake AS, de Silva HJ. Effects of pollution on health of residents in an industrial area in Sri Lanka. Archives of Environmental Health 2002; 57(6): 579-583.
13. Zejda JE, Jarosińska D, Biesiada M, Łaczyński J, Jaźwiec-Kanyion B, Złotkowska R et al. Results of the health survey of a population living in a vicinity of a large waste site (Warsaw, Poland). Central European Journal of Public Health 2000; 8(4): 238-244.
14. Oyana TJ, Lwebuga-Mukasa JS. Spatial relationships among asthma prevalence, health care utilization, and pollution sources in neighborhoods of Buffalo, New York. Journal of Environmental Health 2004; 66(8): 25-37.
15. Kordysh E, Karakis I, Belmaker I, Vardi H, Bolotin A, Sarov B. Respiratory morbidity in hospitalized Bedouins residing near an industrial park. Archives of Environmental and Occupational Health 2005; 60: 147-155.
16. Bentov Y, Kordysh E, Hershkovitz R, Belmaker I, Polyakov M, Bilenko N et al. Major congenital malformations and residential proximity to a regional industrial park including a national toxic waste site: An ecological study. Environmental Health: A Global Access Science Source. (Online) 2006; 5: 8. Available: http://www.ehjournal.net/content/5/8/1 (Accessed 24 September 2008).
17. Sarov B, Bentov Y, Kordysh E, Karakis I, Bolotin A, Hershkovitz R et al. Perinatal mortality and residential proximity to an industrial park. Archives of Environmental and Occupational Health 2008; 63(1): 17-25.
18. The Jewish Virtual Library. The Kibbutz. (Online) 2007. Available: http://www.jewishvirtuallibrary.org/jsource/Society_&_Culture/kibbutz.html (Accessed 24 September 2008).
19. Torbus O, Kalacinski W. [Effect of air pollution and socioeconomic conditions on the incidence of chronic and recurrent respiratory infections in school children]. Pneumonologia Polska 1989; 57(10-12): 460-465. (In Polish)
20. Nicolai T. Epidemiology of pollution-induced airway disease: urban/rural differences in East and West Germany. Allergy 1997; 52(Suppl38): 26-29; discussion 35-36.
21. Vine MF, Stein L, Weigle K, Schroeder J, Degnan D, Tse CK et al. Effects on the immune system associated with living near a pesticide dumpsite. Environmental Health Perspective 2000; 108(12): 1113-1124.
22. Foltinova J, Schrott-Fischer A, Zilinek V, Foltin V, Freysinger W. Is the trachea a marker of the type of environmental pollution? The Laryngoscope 2002; 112(4): 713-720.
23. Bazeliuk LT, Bekeeva SA. [The morphometric assessment of smears from the nasal mucosa of children in Qaraghandy]. Gigiena i sanitariia 1999; 5: 9-10. (In Russian)
24. Duramad P, Tager IB, Holland NT. Cytokines and other immunological biomarkers in children's environmental health studies. Toxicology Letters 2007; 172(1-2): 48-59.
25. Dalton P. Upper airway irritation, odour perception and health risk due to airborne chemicals. Toxicology Letters 2003; 140-141: 239-248.
26. Tsai HJ, Tsai AC, Nriagu J, Ghosh D, Gong M, Sandretto A. Risk factors for respiratory symptoms and asthma in the residential environment of 5th grade schoolchildren in Taipei, Taiwan. The Journal of Asthma: Official Journal of The Association for the Care of Asthma 2006; 43(5): 355-361.
27. Gehring U, Pattenden S, Slachtova H, Antova T, Braun-Fahrländer C, Fabianova E et al. Parental education and children's respiratory and allergic symptoms in the Pollution and the Young (PATY) study. The European Respiratory Journal: Official Journal of the European Society for Clinical Respiratory Physiology 2006; 27(1): 95-107.
28. Koivurova S, Hartikainen AL, Sovio U, Gissler M, Hemminki E, Järvelin MR. Growth, psychomotor development and morbidity up to 3 years of age in children born after IVF. Human Reproduction 2003; 18(11): 2328-2336.
29. El-Toukhy T, Khalaf Y, Braude P. IVF results: optimize not maximize. American Journal of Obstetrics and Gynecology 2006; 194(2): 322-331.
30. Nicolai T. Pollution, environmental factors and childhood respiratory allergic disease. Toxicology 2002; 181-182: 317-321.
31. von Mutius E, Schmid S, PASTURE Study Group. The PASTURE project: EU support for the improvement of knowledge about risk factors and preventive factors for atopy in Europe. Allergy 2006; 61(4): 407-413.
32. Saha A, Kulkarni P, Saiyed H. Living environment and self assessed morbidity: a questionnaire-based survey. BMC Public Health 2007; 7: 223.
33. Pukkala E, Pönkä A. Increased incidence of cancer and asthma in houses built on a former dump area. Environmental Health Perspectives 2001; 109(11): 1121-1125.
34. Ware JH, Spengler JD, Neas LM, Samet JM, Wagner GR, Coultas D et al. Respiratory and irritant health effects of ambient volatile organic compounds. The Kanawha County Health Study. American Journal of Epidemiology 1993; 137(12): 1287-1301.
35. Shohat T, Green MS, Davidson Y, Livne I, Tamir R, Garty BZ. Differences in the prevalence of asthma and current wheeze between Jews and Arabs: results from a national survey of schoolchildren in Israel. Annals of Allergy, Asthma and Immunology 2002; 89(4): 386-392.
36. Kivity S, Sade K, Abu-Arisha F, Lerman Y, Kivity S. Epidemiology of bronchial asthma and chronic rhinitis in schoolchildren of different ethnic origins from two neighboring towns in Israel. Pediatric Pulmonology 2001; 32(3): 217-221.
37. Bjornson CL, Mitchell I. Gender differences in asthma in childhood and adolescence. Journal of Gender-specific Medicine 2000; 3(8): 57-61.
38. Almqvist C, Worm M, Leynaert B, working group of GA2LEN WP 2.5 Gender. Impact of gender on asthma in childhood and adolescence: a GA2LEN review. Allergy 2008; 63(1): 47-57.
39. Xu B, Järvelin MR, Pekkanen J. Prenatal factors and occurrence of rhinitis and eczema among offspring. Allergy 1999; 54(8): 829-836.
40. Kawano Y, Morikawa M, Watanabe M, Ohshiba A, Noma T, Odajima H. A study of the factors responsible for the development of allergic diseases in early life. Asian Pacific Journal of Allergy and Immunology 2005; 23(1): 1-6.
41. Zutavern A, von Klot S, Gehring U, Krauss-Etschmann S, Heinrich J. Pre-natal and post-natal exposure to respiratory infection and atopic diseases development: a historical cohort study. Respiratory Research 2006; 7: 81.
42. Williams FL, Ogston SA. Identifying populations at risk from environmental contamination from point sources. Occupational and Environmental Medicine 2002; 59(1): 2-8.







