Background
To many who work in biopreparedness, the advent of the H1N109 swine influenza pandemic did not come as a surprise. Australian health services have been actively engaged in developing pandemic plans and conducting field exercises for some years1-3. One of the key motivators has been the potential risk posed by the highly virulent but poorly transmissible H5N1 avian influenza strain, which has been circulating globally for more than a decade and has a reported fatality rate among confirmed cases exceeding 60%4. Planning has focused on a worst-case scenario and, thus, the comparatively more moderate infection reported in H1N109 cases meant some incongruence between the perceived level of threat and the public health response.
The inconvenience of social distancing measures and the potential economic impact attracted criticism from the public, media and some sectors of the health community, and there were calls for allowing the pandemic to run its course5-7. However, it must be recognised that Australia was among the first affected countries in the world and soon posted one of the highest infection rates. Unlike North America and Europe, Australia was rapidly heading into its peak winter influenza season. Criticism of its public health response has to be tempered against the fact that little sound epidemiologic information was available when Australia's first cases were identified. Indeed, early data from Mexico suggested a mortality rate that warranted stringent containment measures.
H1N109 Swine influenza
The WHO declared a public health event of international importance on 24 April 2009 in recognition of human transmission of the novel influenza strain, H1N1098. Public health units (PHUs) in Australia were instructed to actively seek cases and apply containment measures, including home isolation/quarantine of confirmed cases and high risk contacts. Antiviral drugs from the national medical stockpile were used to treat cases and reduce the period of infectivity, and also for prophylaxis of high risk contacts. The containment response built on experience gained through field pandemic exercises conducted at Commonwealth, state and area health service level1-3.
The first confirmed Australian swine influenza case arrived in Brisbane on 7 May 2009 on an international flight; by the end of the month 306 cases had been identified across the nation. Local Australian transmission was identified in early June 2009. Global figures reported by WHO showed a 4.4-fold increase in confirmed cases during June 2009 from 17 410 to 77 201, while in Australia, there was a 13.4-fold increase to 4090 confirmed cases over the same period. The disparity between these rates may be related to various factors, including surveillance, laboratory capacity and the progression of the epidemic but there may be other unrecognised explanations. The introduction of a novel influenza strain into countries in the southern hemisphere at the onset of their usual influenza seasons was considered a particular challenge. In Australia the peak influenza period is between July and September, when social factors such as more activities conducted indoors results in crowding and increases the risk of transmission, and low temperatures and humidity aid survival of the influenza virion9.
Reports from North America, including Mexico, provided valuable epidemiological data10-12. The mortality rate of 1.1% reported from Mexico at the early stage of the outbreak was probably inflated by surveillance artefacts and biased towards recognition of cases exhibiting more severe disease. Estimations suggest that the H1N109 virus has a high propensity for transmission with a R0 of 1.4-3.5 compared with 1.2-1.4 for seasonal influenza13. Fifty to 80% of severe cases have had underlying conditions, including pregnancy, asthma or other lung pathology, cardiovascular disease, diabetes, immunosuppression and neurological disorders14,15. Extreme obesity is also being investigated as a potential risk factor16. Severe cases and deaths have occurred in young and previously healthy adults, and less often in children.
The Protect Phase
By mid-June 2009 there was widespread transmission in Victoria and this picture was starting to become evident in New South Wales (NSW), largely in western Sydney and south-western NSW bordering Victoria17. Infection rates varied widely across the country (Table 1) and also within states such as NSW (Table 2). On 4 June, Victoria reported 521 confirmed cases, principally from Melbourne, and this increased to 1011 by 8 June. On 17 June, the Australian Commonwealth's Department of Health and Ageing introduced the 'Protect Phase' across all states, although some parts of Queensland remained in the Contain Phase beyond this date. The Protect Phase focuses on identifying and actively managing vulnerable people with suspected swine influenza infection17. At this stage, testing to confirm H1N1 infection was restricted to people hospitalised for possible influenza.
Table 1: Confirmed H1N109 infection rates in Australian states and territories at the end of the Contain Phase, 17 June 2009
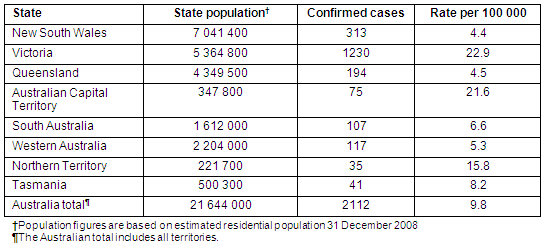
Table 2: Confirmed H1N109 infection rates in the eight New South Wales area health services at the end of the Contain Phase, 17 June 2009
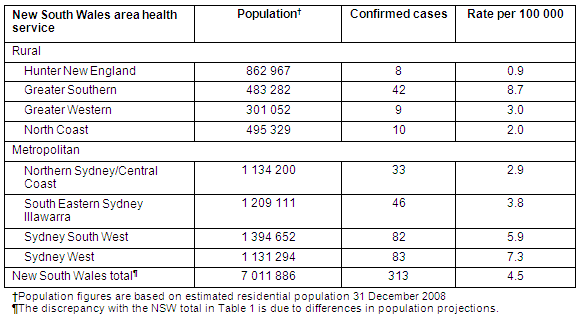
During the Containment Phase considerable effort was made to actively identify cases. Media coverage advised symptomatic people with possible swine influenza risk exposures to seek medical assistance. Information was circulated to GPs and emergency departments regarding the clinical and epidemiological recognition of swine influenza and doctors were encouraged to contact their local PHU if a suspected case presented. More than 2000 people were tested in NSW alone. Data recorded in Tables 1 and 2 suggest considerable areas of Australia were spared large-scale introduction or were successful in containing the early spread of the disease, although surveillance is unlikely to capture all cases of H1N109. The heterogeneous spread of swine influenza also reflects the experience of previous pandemics, and provides further motivation for surging public health resources to bolster local containment18. In addition, it is appropriate to share resources with more affected areas in order to sustain containment, particularly when local capacity is compromised.
Do containment strategies provide long-term benefit?
When the Protect Phase was declared, case rates were less than 9/100 000 for most areas of Australia, except Victoria and the Australian Capital Territory which were 22-23/100 000. This raises the question of whether it was appropriate for all Australian regions to terminate their containment strategies simultaneously when many PHUs appeared to be effectively controlling transmission? A variety of factors need to be considered in the decision, including the value of persevering with containment in the face of escalating transmission in neighbouring areas, the cost of enforcing quarantine and social distancing, the ability to surge laboratory capacity and maintain other essential diagnostic services, the virulence and clinical impact of the influenza strain, the effectiveness and availability of antiviral treatment, and the timeframe for developing a targeted vaccine.
In a country as large as Australia with natural barriers of distance and geography, it is reasonable to expect that some areas can be isolated from the impact of a novel infectious disease, even if wide-scale activity is occurring elsewhere. Reducing the spread of the novel virus is in part dependent on people complying with social distancing measures, and there is evidence that Australians will cooperate with public health requests19. As only rare cases of antiviral resistance to H1N109 have been observed, treatment and prophylaxis must be regarded as effective control measures in this instance20.
Two weeks after the introduction of the Protect Phase the number of confirmed cases in Australia doubled, despite confirmatory testing (and hence surveillance) only being focused on severe cases. In NSW, 10 cases were hospitalised in the Containment Phase and 187 in the following 2 weeks. Approximately 20% of those hospitalised have required treatment in an intensive care unit21. The first H1N109-associated death was reported from South Australia on 19 June and the toll has steadily increased. These statistics suggest that H1N109 influenza will result in many cases of severe disease when there is widespread community infection, an argument for containment if it could have been sustained. Similarly, rigorous containment measures are appropriate to protect vulnerable individuals and communities. This includes people with underlying medical conditions and also Indigenous Australians, a group which historically has borne a heavy burden during introductions of novel influenza infections22. Statistics indicate that Indigenous people are approximately five times more likely than non-Indigenous Australians to be hospitalised for swine influenza21. Currently (1 September 2009), the cumulative hospitalisation figures indicate that there have been 4440 swine influenza admissions to Australian hospitals, with 13.8% being Indigenous Australians, and at least 20 of the 154 people who have died with confirmed H1N109 infection are known to be Indigenous21. The proportion of people identifying as Indigenous in the Australian population is 2.5%23.
Rural experiences
During the Containment Phase many towns in rural and remote parts of Australia were spared from swine influenza. Our experience dealing with GPs from country areas suggests that they were enthusiastically engaged in active case ascertainment and assisted public health authorities with the implementation of control measures. Many were reluctant to accept the relaxed measures described in the Protect Phase guidelines24. Furthermore, their intimate local knowledge often provided the effective surveillance necessary for successful containment. A particular concern for managing large numbers of pandemic cases once established in rural areas is the issue of inequitable access to health services and the well recognised shortage of medical officers25. In addition, delays in providing confirmation of cases from country towns were evident during the Containment Phase because of specimen transportation difficulties and laboratory turnaround times. The GPs in these areas may have to rely more heavily on clinical acumen to recognise cases and encourage isolation before pathology results are available.
Vaccines
The principal measure for controlling viral infections is comprehensive coverage with an effective vaccine. In the case of influenza, this has necessitated annual development of a vaccine tailored to the forecasted seasonal strains and derived from viral antigen cultured in eggs. While the influenza vaccine is generally effective, the limitations are obvious when rapid production is required for a novel influenza strain. It can take months to develop a suitable vaccine and further delays are experienced in confirming safety and efficacy through clinical trials. In addition, an effective immune response may require two doses. For some countries the vaccine may be ready as soon as mid-September 200914; however, it is important that the public has confidence in its safety and that full therapeutic goods registration is obtained before it is made available. In the future, cell-line derived and genetically engineered vaccines may significantly reduce the period of time to develop a strain-specific vaccine26. During the swine influenza response it is possible that some areas could have maintained containment until the H1N1 vaccine was available, and this could have mitigated the impact of the novel virus, but such a strategy needs to be weighed against the increased cost, social disruption, and demand on the local health workforce.
Conclusion
Although containment measures were universally applied across Australia, their impact during the initial response to the H1N109 swine influenza pandemic was diverse. It is debatable whether the Australian health sector could have maintained the intense containment approach for long enough to preserve all areas from the affects of community wide transmission. However, a compelling argument can be lodged for an approach of maintaining containment in unaffected areas in future pandemic responses, particularly in country areas where access to health care may be problematic and there is a high proportion of at-risk individuals, including Aboriginal and Torres Strait Islanders.
In a country the size of Australia, disease patterns are influenced by a multitude of factors including population density, demographics, cultural traditions and behaviours, transport routes, geographical barriers and health service capacity. Thus, heterogeneous application of containment measures using an 'area quarantine' approach should be included in pandemic plans for future occasions when community transmission affects certain parts of the country but spares others. A heterogeneous approach could decrease the inherent inequities of an approach of managing only individuals at higher risk of complications. Area quarantine would be particularly appropriate for a virulent infectious agent where the overall aim is to reduce morbidity and mortality.
References
1. Australian Government Department of Health and Ageing. National Pandemic Influenza Exercise, Exercise Cumpston 06 Report. (Online) 2009. Available: http://www.health.gov.au/internet/panflu/publishing.nsf/Content/34B24A2E2E6018E9CA2573D7000006D2/$File/exercise-cumpston-report.pdf (Accessed 1 September 2009).
2. Craig AT, Armstrong PK. Exercise Paton: A simulation exercise to test New South Wales emergency departments' response to pandemic influenza. Communicable Diseases Intelligence 2007; 31: 310-313. Available: http://www.health.gov.au/internet/main/publishing.nsf/Content/cda-cdi3103-pdf-cnt.htm/$FILE/cdi3103i.pdf (Accessed 1 September 2009).
3. Hunter New England Population Health. Hunter New England XFG Pandemic Influenza Exercise, 22-26 September 2008, Interim Exercise Report December 2008. (Online) 2009. Available: http://www.hnehealth.nsw.gov.au/__data/assets/pdf_file/0017/53180/HNEH_XFG_Final_Summary_Report_Dec_2008.pdf (Accessed 1 September 2009).
4. WHO. Cumulative Number of Confirmed Human Cases of Avian Influenza A/(H5N1) Reported to WHO. (Online) 2009. Available: http://www.who.int/csr/disease/avian_influenza/country/en/ (Accessed 1 September 2009).
5. Robinson N. Just when we all thought it was a hoax of World Health Organisation Level 6 pandemic proportions, Australia is in the grip of swine flu and nobody seems to care. (Online) May 28, 2009. The Australian. Available: http://www.theaustralian.news.com.au/story/0,,25547677-25090,00.html (Accessed 1 September 2009).
6. Collignon P. Take a deep breath, Swine flu's not that bad. (Online) 25 May 2009. Crikey. Available: http://www.crikey.com.au/2009/05/25/take-a-deep-breath-swine-flus-not-that-bad/ (Accessed 1 September 2009).
7. Barlow K. Let swine flu run its course: expert. ABC News, Lateline. (Online) 2009. Available: http://www.abc.net.au/news/stories/2009/05/29/2585109.htm (Accessed 1 September 2009).
8. WHO. Influenza-like illness in the United States and Mexico, 24 April 2009. (Online) 2009. Available: http://www.who.int/csr/don/2009_04_24/en/index.html (Accessed 1 September 2009).
9. Lowen AC, Mubareka S, Steel J, Palese P. Influenza virus transmission is dependent on relative humidity and temperature. Public Library of Science Pathogens 2007; 3: 1470-1476. (Online) 2009. Available: http://www.plospathogens.org/article/info:doi/10.1371/journal.ppat.0030151 (Accessed 1 September 2009).
10. Centers for Disease Control and Prevention. Swine-origin influenza a (h1n1) virus infections in a school, New York City. (Online) 2009. Available: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5817a6.htm (Accessed 1 September 2009).
11. Chowell G, Bertozzi SM, Colchero MA, Lopez-Gatell H, Alpuche-Aranda C, Hernandez M et al. Severe respiratory disease concurrent with the circulation of h1n1 influenza. New England Journal of Medicine. (Online) 2009. Available: http://content.nejm.org/cgi/reprint/NEJMoa0904023v1.pdf (Accessed 1 September 2009).
12. Perez-Padilla M, Rosa-Zamboni D, Ponce de Leon S, Hernandez M, Quiñones-Falconi F, Bautista E et al. Pneumonia and respiratory failure from swine-origin influenza A (H1N1) in Mexico. New England Journal of Medicine 2009; 361: 680-689. Available: http://content.nejm.org/cgi/content/full/NEJMoa0904252 (Accessed 1 September 2009).
13. Hiroshi Nishiura H, Wilson N, Baker MG. Estimating the reproduction number of the novel influenza A virus (H1N1) in a Southern Hemisphere setting: preliminary estimate in New Zealand. The New Zealand Medical Journal 2009; 122. Available: http://www.nzma.org.nz/journal/122-1299/3722/ (accessed 1 September 2009).
14. WHO. Pandemic (H1N1) 2009 briefing note 4: preliminary information important for understanding the evolving situation. (Online) 2009. Available: http://www.who.int/csr/disease/swineflu/notes/h1n1_situation_20090724/en/print.html (Accessed 1 September 2009).
15. Jamieson DJ, Honein MA, Rasmussen SA et al. H1N1 2009 influenza virus infection during pregnancy in the USA. (Online) 2009. The Lancet 2009; 374(9688): 451-458. Available: http://www.thelancet.com/journals/lancet/article/PIIS0140-6736(09)61304-0/fulltext (Accessed 1 September 2009).
16. Centers for Disease Control and Prevention. Intensive-care patients with severe novel influenza a (h1n1) virus infection - Michigan. (Online) 2009. Available: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm58d0710a1.htm (Accessed 1 September 2009).
17. NSW Government, Department of Health. Media Release from the Minister for Health, John Della Bosca, 17 June 2009. (Online) 2009. Available: http://www.emergency.health.nsw.gov.au/swineflu/news/2009/20090617_00.html (Accessed 1 September 2009).
18. Miller MA, Viboud C, Balinska M, Simonsen L. The signature features of influenza pandemics, implications for policy. (Online) 2009. New England Journal of Medicine 2009; 360: 2592-2598. Available: http://content.nejm.org/cgi/content/full/360/25/2595 (Accessed 1 September 2009).
19. Eastwood K, Durrheim D, Francis JL, d'Espaignet T, Duncan S, Islam F et al. Knowledge about pandemic influenza and compliance with containment measures among Australians. Bulletin of the World Health Organisation 2009; 87. (Online) 2009. Available: http://www.who.int/bulletin/volumes/87/8/08-060772/en/index.html (Accessed 1 September 2009).
20. Bullock L. WHO identifies viruses resistant to Oseltamivir. Elsevier Global Medical News 2009. (Online) 2009. Available: http://www.thelancet.com/H1Nv1-flu/egmn/0c03a65d (Accessed 1 September 2009).
21. Australian Government Department of Health and Ageing. Australian influenza surveillance 2009. (Online) 2009. Available: http://www.healthemergency.gov.au/internet/healthemergency/publishing.nsf/Content/ozflucurrent.htm (Accessed 1 September 2009).
22. Curson P, McCraken K. An Australian perspective of the 1918-1919 influenza Pandemic. NSW Public Health Bulletin 2006; 17: 103-107. Available: http://www.publish.csiro.au/?act=view_file&file_id=NB06025.pdf (Accessed 1 September 2009).
23. Australian Bureau of Statistics. Australia's Population. Available: http://www.abs.gov.au/AUSSTATS/abs@.nsf/Web+Pages/Population+Clock?opendocument?utm_id=LN (Accessed 1 September 2009).
24. NSW Health. Response to human swine influenza: Protect Phase (Online) 2009. Available: http://www.emergency.health.nsw.gov.au/swineflu/resources/pdf/protect_phase.pdf (Accessed 1 September 2009).
25. Australian Institute of Health and Welfare. Medical labour force 2004, National Health Labour Force Series no 38; cat. no. HWL 39. Canberra: AIHW, 2006. (Online) 2009. Available: http://www.aihw.gov.au/publications/hwl/mlf04/mlf04.pdf (Accessed 1 September 2009).
26. University of Queensland. UQ generates first Australian swine flu vaccine. 2009. (Online) 2009. Available: http://www.uq.edu.au/news/index.html?article=18698 (Accessed 1 September 2009).
Abstract
Recent experience during Australia's initial public health response to the swine influenza pandemic provides valuable lessons for the future. An intense containment effort lasting 7 weeks was unable to prevent local community transmission in some areas of Australia; however, despite the mobility of many people living in rural and remote parts of the country, much of the outback was unaffected. By the end of the Containment Phase, most parts of rural New South Wales only recorded low rates of confirmed H1N109 infection. As Australians living in rural areas often have poorer access to health services than their urban counterparts, they are likely to be more affected by an extended emergency, even one as moderate as the present H1N109 swine influenza pandemic. There may have been benefits in extending containment measures in these less affected areas and in communities where large numbers of vulnerable people such as Indigenous Australians reside. Containment is worthwhile in limiting the spread of disease in specific situations but is unlikely to change the course of a pandemic unless it can be sustained until a large proportion of the population is vaccinated. Strenuous containment efforts should certainly be applied in outbreaks of severe disease, particularly those caused by novel infectious agents with a low reproductive rate (R0). Should advances in vaccine manufacture reduce the time taken to produce a new vaccine, then increased effort to extend containment will be even more worthwhile.
Key words: Australia, H1N1, human influenza, pandemic, swine influenza, transmission, prevention and control.



