Introduction
Health care and research in rural populations are often limited due to a lack of infrastructure and small sample sizes1. Nonetheless, such populations have a need for medical care and can be of great value when studying the health effects of lifestyle and genetic factors. For example, the northern parts of Norway, Sweden, Finland and the Kola Peninsula (collectively called the Northern Shield) are affected by both climate and cultural changes. These cause a transition from a traditional, subsistence-based lifestyle to a modern, industrialized lifestyle for many local people2 Their traditional lifestyle is based on reindeer herding, fishing, and hunting, but an increasing number of people now have other occupations and, therefore, have other diet and activity levels3. The effect of this transition on disease prevalence is at present unknown and requires special medical attention4. Therefore, there is a need to focus on the health conditions of rural populations on the northern periphery of major continental areas.
At the same time as rural populations in Nordic regions undergo lifestyle changes, many common diseases are spreading with epidemic proportions in most urban populations with a modern, industrialized lifestyle5. Although major international efforts are underway to identify environmental and genetic factors relevant to disease development by establishing very large prospective cohorts in metropolitan populations6,7, a major difficulty lies in the complexity of the environmental and genetic factors relevant for common disease. Because this complexity is typically reduced in rural compared with urban populations (Table 1), studies of such populations have a higher potential for determining risk factors related to common disease than urban populations8-12.
Table 1: Characteristics of traditional versus modern lifestyles: examples comparing a traditional lifestyle in Northern Sweden with a modern lifestyle in Western Europe
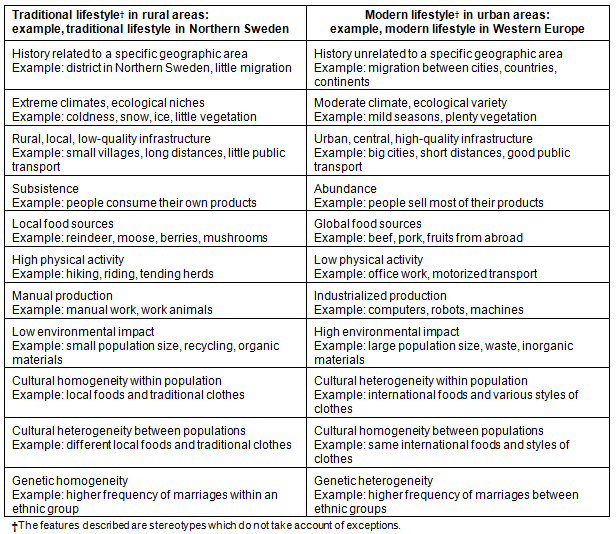
Variation in environmental factors, for example climate or diet, is typically reduced in rural relative to urban populations8. If risk factors for medical traits are examined, these homogenous conditions create a natural study design controlling other influences and reducing the 'noise' (confounders) compared with the 'signal' (risk factor)13. A second consideration is that lifestyles between rural and urban populations can be very different14. This will maximize the health effects of lifestyle factors in comparative studies making them easier to detect. In addition, rural individuals usually live in the same environment over an extended time period, which again increases the health effects of their lifestyle. If a subgroup within a rural population is changing towards a modern, industrialized lifestyle, the health effects of lifestyles differences can even be compared within the same population. If the lifestyle differences are known and measured, advantage can be taken of similar environmental conditions to control noise and include known environmental differences in the analytical model. This way one can again remove the noise and strengthen the signal. In addition, the effect of the measured environmental confounders and its interactive effects on the examined medical trait can be studied13.
The history and structure of a population also influence the power of mapping genetic factors relevant for disease development. Many rural populations have lived isolated from surrounding populations while either retaining a small and stable population size over a long time, experiencing a recent population bottleneck followed by exponential growth, and/or showing a higher level of consanguinity. All these factors result in lower heterozygosity15, which reduces the overall genomic variation and increases the frequency of rare disease-relevant genotypes16. For example, genes that cause rare monogenic genetic bone diseases (eg osteopetrosis and sclerosteosis), which now represent important molecular targets for the design of new drugs to treat osteoporosis17, were identified in a rural population.
The Northern Sweden Population Health Study (NSPHS) was initiated to provide a health survey of the population in this area and to study the medical consequences of lifestyle and genetics. This article will provide the results on differences between modern and traditionally living individuals regarding lifestyle, subclinical, and clinical measures within the NSPHS study. In the discussion, some of the contributions of this project to past publications of the European Special Populations Research Network (EUROSPAN, http://www.eurospan.org) and other international consortia, which discovered novel genetic determinants on medical traits18-28, will be reviewed.
Methods
Organization
Data collection in a rural population requires special consideration of specific geographical, cultural, and historical conditions beyond usual scientific standards. Therefore, supporting material regarding features of the study the authors found to be important are provided (Appendix I) for the benefit of other researchers planning similar research.
Sample
The study population has been living remote from other influences for a long time due to geographical, historical, political, cultural, and linguistic reasons. Therefore, the population showed closer family relationships than urban populations, and had a genealogy that was well documented in church records, extending up to 300 years. According to the Sweden Census, on 31 December 2006 this parish had 1071 inhabitants of whom 826 (77%) were aged 15 years or older (and therefore eligible for this study). Overall, 740 (90%) of 826 eligible individuals gave a written informed consent. Of the 740 participants of the study, 656 subjects (89%) contributed complete data, resulting in a final sample consisting of 347 (53%) women and 95 (14.5%) individuals with a traditional lifestyle.
The NSPHS study was approved by the local ethics committee at the University of Uppsala (Regionala Etikprövningsnämnden, Uppsala, Dnr 2005:325) in compliance with the Declaration of Helsinki29. All participants gave their written informed consent to the study. For participants of under legal age, a legal guardian also signed. The procedure used to obtain informed consent and the respective informed consent form has been recently discussed according to current ethical guidelines30.
Measures
The study included a comprehensive collection of data on genealogy, sociodemography, body size, blood samples for clinical chemistry, medical history of participants and family members, and lifestyle. The following describes only measures with relevance to cardiovascular, metabolic, and orthopedic disease.
Genealogy, sociodemography, and body size: Assessed were genealogy, sociodemography (eg sex, age, occupation) and body size measures (eg height, weight, hip circumference, and body mass index). Individuals were grouped into 4 groups based on self-reported information on sex and occupation, distinguishing individuals reporting reindeer herding as their major occupation versus all other occupations. Individuals reporting reindeer herding as their major occupation performed related activities (eg tending herds, processing reindeer meat and fur, buying and selling reindeer or reindeer products) on a regular, daily basis, and showed other lifestyle characteristics, for example high mobility and seasonality of work. Unclear cases were discussed with the district nurse who knew most of the community members and their families. These four subgroups with different sex and occupations/lifestyles are referred to as: traditional lifestyle (TLS) men, TLS women, modern lifestyle (MLS) men, and MLS women.
Lifestyle:
Diet Data were collected with a food frequency questionnaire based on the Northern Sweden 84-item Food Frequency Questionnaire (NoS-84-FFQ). This food frequency questionnaire was developed, validated, and applied within the Västerbotten Intervention Program31-33. The answer options were in an 11 point format: 0='Never', 1='less than 1 time per month', 2='1 to 3 times per month', 3='1 time per week', 4='2 to 4 times per week', 5='5 to 6 times per week', 6='1 time per day', 7='2 to 3 times per day', 8='4 to 5 times per day', 9='6 to 8 times per day', 10='9 to 10 times per day'. For each food item, daily intake was calculated in grams per day as a standardized unit of measurement and the items aggregated to food categories, such as game meat, non-game meat, fish, dairy products, vegetables, or fruit. Food intake was converted to nutrients (eg fibre, carbohydrates, protein, fat, and alcohol) and their energy equivalent using the food database of the Swedish Food Administration34.
Included in the questionnaire were several items on foods specific for the lifestyle in this geographic region, in particular on game consumption (reindeer, moose). The construct validity (known-groups validity) of the items on game consumption added to the NoS-84-FFQ questionnaire was evaluated. Traditional (n=94) versus modern lifestyle (n=505) individuals were compared. A highly significant, large effect size (ES) in men (ES=(MTLS-MMLS)/SDpooled=1.25, p=9.7×10-04) and women (ES=1.15, p=2.9x10-05) was observed in the expected direction, corresponding to an approximately three times higher consumption of absolute overall game intake in those with a traditional lifestyle.
Physical activity Two self-report scales were used to measure overall physical activity at work and at leisure. The Work Activity Scale (WAS, 6 items) addressed typical occupational physical activities: sitting, standing, walking, lifting, and general indicators of physical activity, that is sweating and tiredness after work. The Leisure Activity Scale (LAS, 4 items) asked for various typical free-time activities, such as walking, cycling, other sporting activities, and sweating as a general indicator of physical activity. Participants reported the frequency of each activity on a 5 point rating scale (1='never', 2='seldom', 3='sometimes', 4='often', and 5='always'). Both scales showed satisfying internal consistency with Cronbach's α(WAS)=0.73 and Cronbach's α(LAS)=0.70.
Other health risks: Participants were asked about their consumption of cigarettes and snuff (a regional form of tobacco consumption where tobacco infusion is retained in the mouth) and whether they had ever experienced a work or traffic accident. Answers were given in a dichotomous format (0='no', 1='yes').
Subclinical measures
Blood measures: Resting pulse rate and systolic and diastolic blood pressure were measured. Total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and triglycerides (TG) in the blood serum were quantified by enzymatic photometric assays using an ADVIA1650 clinical chemistry analyzer (Siemens Healthcare Diagnostics GmbH; Eschborn, Germany) at the Institute for Clinical Chemistry and Laboratory Medicine, Regensburg University Medical Center, Germany. Sphingolipids and phospholipids in the blood plasma were quantified by electrospray ionization tandem mass spectrometry (ESI-MS/MS) in positive ion mode as described previously35-37.
Lung function: Spirometry was performed in a sitting position without noseclips using a Spida 5 spirometer (MicroMedical; http://www.medisave.co.uk). Three consecutive lung function measurements per participant were performed and the maximum value per measured lung function parameter was used for further analysis. Within the scope of this article, Peak Expiratory Flow (PEF), Forced Expiratory Volume (FEV), and Forced Vital Capacitiy (FVC) were analyzed.
Bone mineral density: Bone status was assessed at the heel using the Lunar Achilles Express ultrasonometer (GE Healthcare; http://www.gehealthcare.com). Data are presented on the stiffness index as a Z-scaled measure of bone mineral density.
Clinical measures
Participants were asked during a clinical interview whether they suffered from symptoms or diseases of the cardiovascular, metabolic system or musculoskeletal system (eg pain during the last week) at present or had in the past (Table 5). The district nurse coded the reported symptoms and diseases in the relevant categories and asked for additional information if the self-report was unclear.
Statistical analysis
Primarily descriptive, comparative results are presented for relevant subgroups in the sample (Men: modern vs traditional lifestyle; Women: modern vs traditional lifestyle). Sample size independent effect size measures were calculated to evaluate the strength of the effects for quantitative (ESquantitative=(MTLS -MMLS)/SDpooled) and binary (ESbinary= PercentageTLS - PercentageMLS) traits. According to a rule of thumb, ESquantitative values of approximately 0.2 were considered small, approximately 0.5 medium, and approximately 0.8 as large38.
Also provided are inferential results per outcome. However, the authors are aware of the limited power of this sample and study design for hypothesis testing. Therefore, it is stressed that a lack of statistical significance does not prove that there is no relevant difference between the subgroups39.
Nominal statistical significance was based on a local type I error of α=0.05; α inflation caused by multiple testing was not allowed for because conventional approaches are biased if applied to correlated outcomes (eg diet, activity, foods, lipids), as in this case. Instead this issue was addressed by reporting all conducted statistical tests40, leaving the overall evaluation of statistical significance to the reader.
Because the data originates from a pedigree-based sample, adjustments for correlated data were made to derive the correct statistical inference41. A pedigree-based sample consists of individuals from a population where a substantial number of persons belong to the same extended families. This means that many participants are related to each other to varying degrees, but much closer than a sample from a general population-based sample. Therefore, a generalized linear mixed effects model was applied to estimate the effect of lifestyle on various outcomes in the male and female subsample. Deviations from normality of quantitative traits (eg body measures, diet, physical activity, and lipid levels) were corrected by inverse-normal transformation. Binary outcomes remained untransformed. The analysis was performed using the statistical analysis system R (V2.8.1)42.
Results
Body measures
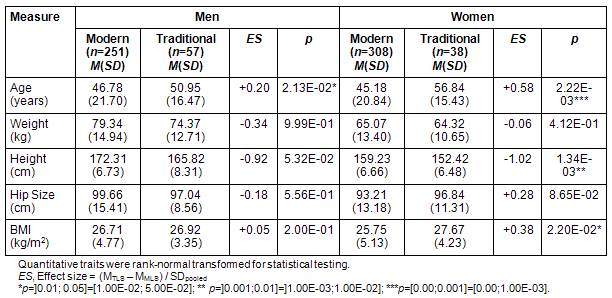
Participants were compared according to traditional (n=95) and modern (n=559) lifestyle separately in men (n=308) and women (n=346), regarding body size, subclinical, and clinical measures. TLS men were on average 4 years older (p=0.021) and TLS women on average 12 years older (p=0.0022) than the corresponding MLS groups. There were no substantial mean differences in weight for men and women, but TLS women were on average 6.8 cm shorter (p=0.0013), resulting also in a 2 kg/m2 higher Body Mass Index (p=0.022) for TLS women (Table 2).
Table 2: Body measures in modern living men and women and those living traditionally
Lifestyle
Diet: Men and women with a traditional lifestyle consumed on average approximately 50% less non-game meat (Men: 87.95 vs 42.03 g/day, p=1.4×10-07; Women: 81.03 vs 42.06 g/day, p=0.026) and milk products (Men: 581.22 vs 298.93 g/day, p=0.0011; Women: 430.83 vs 360.15 g/day, p>0.05) compared with those with a modern lifestyle. However, TLS men (71.02 vs 193.63 g/day, p=0.0011) and women (56.42 g/day vs 139.92 g/day, p=0.0020) consumed approximately 3 times more game meat than MLS subjects. Participants with a traditional lifestyle consumed less vegetables (Men: 59.05 vs 53.17 g/day; p>0.05; Women: 100.00 vs 71.77 g/day, p=0.042), but their intake of fruit (including berries) was higher, although these differences did not reach nominal significance (Table 3).
Table 3: Self-reported lifestyle in modern living men and women and those living traditionally
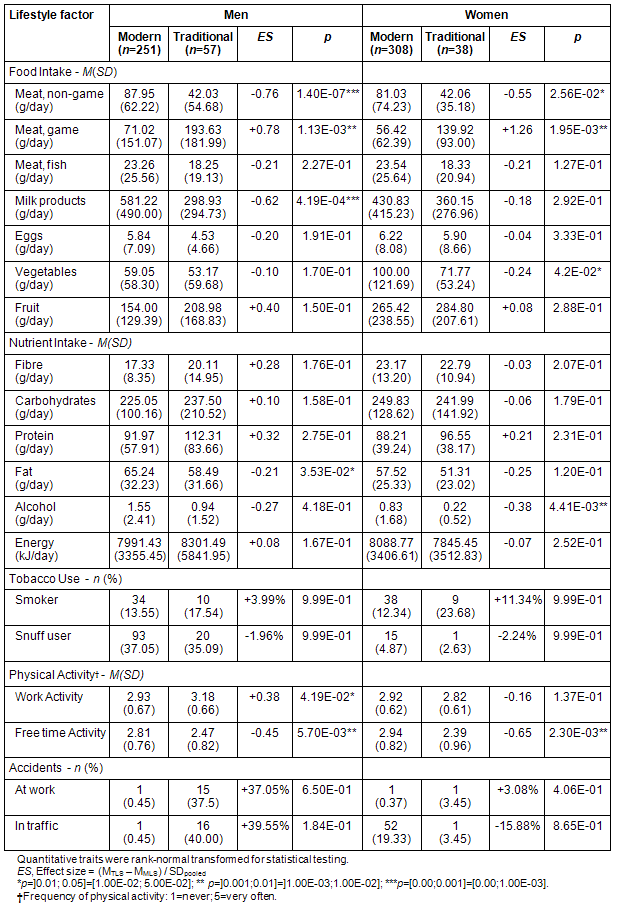
Among the macronutrients, fat (Men: 65.24 vs 58.49 g/day, p=0.035; Women: 57.52 vs 51.31 g/day, p>0.05) and alcohol intake (Men: 1.55 vs 0.94 g/day, p>0.05; Women: 0.83 vs 0.22, p=0.0044) were both substantially lower in TLS subjects. Intake of protein was higher in TLS men and women, although this difference was not significant. The total energy intake based on self-reported food intake was higher in TLS men and lower in TLS women, which corresponds to self-reported physical activity at work (all p>0.05, Table 3).
Other health-related behavior: Smoking and using snuff showed minor variations between lifestyles, apart from smoking which was more frequent in traditionally than modern living women (12.34% vs 23.68%, all p>0.05, Table 3).
TLS men showed increased physical activity at work (p=0.042) but decreased activity at leisure (p=0.0057) compared with MLS men. TLS women, however, reported similar, but lower physical activity at work (p>0.05) and substantially less activity in their free time (p=0.0023, Table 3).
TLS men showed a dramatically higher frequency of working accidents (0.45% vs 37.50%) and traffic accidents (0.45% vs 40.00%) compared with MLS men. Traditionally living women had similar rates of work accidents (3.45% vs 0.37%) but much lower rates of traffic accidents (19.33% vs 3.45%, all p>0.05, Table 3).
Subclinical measures
Blood circulation: Pulse rates of TLS participants were moderately higher in men (71.89 vs 74.28 bpm) and women (71.95 vs 74.62 bpm). Blood pressure levels were similar among men, but systolic (120.08 vs 124.74 mmHg) and diastolic (72.93 vs 73.95 mmHg) values tended to be higher in TLS women (all p>0.05, Table 4).
Table 4: Objective, subclinical health measures in modern living men and women and those living traditionally
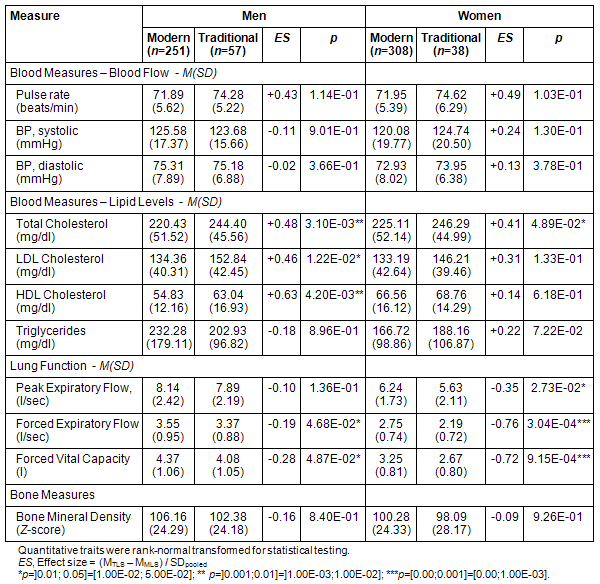
Blood lipids: Substantially elevated cholesterol levels (TC, LDL-C, and HDL-C) were found in male and female TLS participants. The differences for TC (Men: 220.43 vs 244.40 mg/dl, p=0.0031; Women: 225.11 vs 246.29 mg/dl, p=0.049) and LDL-C (Men: 134.36 vs 152.84 mg/dl, p=0.012; Women: 133.19 vs 146.21 mg/dl, p=ns) and HDL-C (Men: 54.83 vs 63.04 mg/dl, p=0.0042; Women: 66.56 vs 68.76 mg/dl, p>0.05) were of small to medium size. However mean total cholesterol levels of male and female MLS subjects were 220 and 225 mg/dl, respectively, which indicated a borderline high cardiovascular risk; and total cholesterol levels of TLS males and females were 244 mg/dl (men) and 246 mg/dl (women) on average, suggesting high risk43. On an individual level, this implied that among MLS participants 34% (195 of 561) fell into the high risk group, while 63% (61 of 97) of TLS subjects were exposed to a similar degree. Triglyceride levels also showed small differences between MLS and TLS men (232.28 vs 202.93 mg/dl) and women (166.72 vs 188.16 mg/dl), without reaching statistical significance (Table 4).
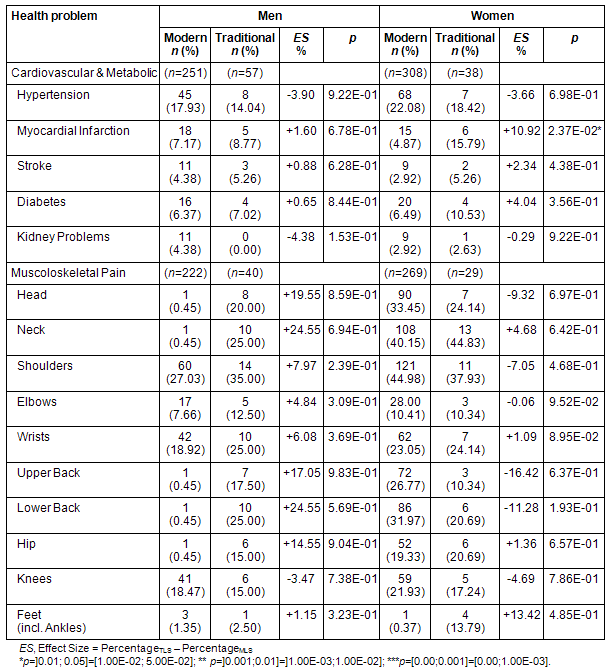
Table 5: Self-reported health problems in modern living men and women and those living traditionally
Lung function: All lung function measures (peak expiratory flow, forced expiratory flow, forced vital capacity) showed lower values for TLS compared with MLS participants, with nominally significant differences (all p<0.05) for all measures and sexes, except PEF in men, and were highly significant differences in women (Table 4).
Bone mineral density: The Z-scaled stiffness index for measuring bone mineral density showed somewhat lower values for TLS men (106.16 vs 102.38) and women (100.28 vs 98.09) but the values were not significantly different (Table 4).
Clinical measures
Cardiovascular and metabolic: Prevalence for various cardiovascular and metabolic diseases (hypertension, stroke, diabetes, kidney problems) showed only minor differences between MLS and TLS participants in men and women. However, a nominally significant increase of myocardial infarction was observed in TLS women (4.87% vs 15.79%, p=0.024, Table 5).
Musculoskeletal: TLS men consistently reported pain more often in various body parts during the week before the examination, with the strongest differences in head (0.45% vs 20.00%), neck (0.45% vs 25%), upper back (0.45% vs 17.50%), lower back (0.45% vs 25.00%), and hip (0.45% vs 15.00%). TLS women, on the contrary, did not report consistently more pain than MLS women. The largest differences existed in TLS women reporting less pain in the head (33.45% vs 24.14%), upper back (26.77% vs 10.34%), and lower back (31.97% vs 20.69%) compared with MLS women. None of the differences proved nominally significant (Table 5).
Discussion
The Northern Swedish Population Health Study (NSPHS) was presented here, a population-representative, cross-sectional study of a rural population in Karesuando, County of Norrbotten, Sweden, in the very north of Sweden north of the Arctic Circle. It is a paradigmatic study in the sense that it attempts to connect two focus areas of research to: (i) provide a survey of the health status and the specific needs of the individuals and the community; and (ii) perform research on novel environmental and genetic determinants of non-communicable diseases on an international level. A summary and discussion follows that present findings on lifestyle differences and health outcomes between modern and traditionally living individuals in the NSPHS study described in this article. Additionally, past contributions of this study to the identification of novel genetic determinants of health relevance in international meta-analysis studies18-28 are reviewed.
Lifestyle and health - a community-health perspective
Traditionally living men consume much more game meat, less non-game meat and less milk products compared with men of a modern life style. Similar differences in consumption of game and non-game meat are found in women, who also seem to consume fewer vegetables. From a nutritional point of view, the difference in game meat consumption can explain the lower intake of fat and the higher intake of protein in the TLS group. These results corroborate a recent study on lifestyle differences (diet, activity) in a more southward population of the Swedish mountain range3.
The differences in game and vegetable intake, especially in women, carry two risk factors that can have direct effects on lipid levels and indirectly also affect cardiovascular risk. Although game meat contains much more protein (27.11% vs 17.32%) and less fat (7.36% vs 12.75%) compared with non-game meat, the cholesterol content is specifically higher in game (0.76% vs 0.60%) compared with pork or beef34. Furthermore, vegetables are a major source of plant sterols, especially β-sitosterol, which specifically inhibit the uptake of cholesterol in the intestines44 and are associated with a lower risk for coronary heart disease45. Interestingly, it was seen that total, LDL, and HDL cholesterol levels are also consistently increased in individuals with a traditional lifestyle, indicating high risk values for total cholesterol values for approximately two out of three individuals43. In agreement with increased cholesterols in TLS, blood pressure tended to be higher in TLS women, and both TLS men and women reported moderately higher pulse rates in contrast to their respective comparison group. A higher prevalence of myocardial infarction in traditionally living women was also seen, an increase that agrees with previous studies46, whereas no substantial differences in prevalence of cardiovascular or metabolic diseases were found in men.
The observed lower intake of dairy products, especially in men, can negatively affect the bioavailability of micronutrients such as calcium or vitamin D, which are essential for bone health47. Heaney et al. even stated that 'it is difficult to devise a diet that is "bone healthy" without including three servings of dairy per day'. Bone mineral density (BMD) of both TLS men and women tended to be lower than in respective MLS comparison groups. The BMD values corresponded well with differences in the intake of dairy products between MLS and TLS men and women, indicating the importance of milk products for bone health.
Traditionally living men seem to be more active at work, but less so during free time than modern living men. Traditionally living women, however, show overall lower physical activity compared with modern living women. Consistently with this higher level of physical activity at work, TLS men also report higher rates of work and traffic accidents than MLS men. An opposite pattern of traffic accidents is found in women, with TLS women reporting fewer problems. The TLS men also reported much higher frequencies of work or traffic related accidents. The fact that opposite effects were observed in the male and female group supports the view that the high frequency in men is due to their high mobility at work. Orthopedic symptoms, in terms of physical pain in various body parts, were increased in men with a more physically demanding lifestyle. However, a similar or lower proportion of pain symptoms were reported in TLS compared with MLS women. The physical location of pain in the body with a high percentage of TLS men reporting pain in the head, neck, back, or hip further strengthens the view that the physical activity of TLS men results in multiple effects. This evidence is supported by qualitative reports on the working life of reindeer herders who describe hard physical labor at cold temperatures and heavy use of snow mobiles. Driving their vehicles at high speed over long distances on bumpy ground to tend their herds causes strong and continuous concussions of the spine. Since their work is highly seasonal this means that there are periods during the year with highly intense work with little time for rest and recreation2.
Individuals with a traditional lifestyle showed consistently lower lung function than individuals in their comparison group, with larger differences in women. Although these differences require careful interpretation because of the body height differences (highly correlated with lung function) between the subgroups, these results suggest a relationship of less physical activity and increased smoking rates with lower lung function in traditionally living women.
The authors are aware of the limitations of this study regarding the reliability (small sample size, sample size imbalances) and validity (confounders) of the presented results. The application of sophisticated, parametric statistical models to small sample sizes may have caused conservative results. However, considering the complicated data structure the authors are not aware of better statistical models or tools specifically developed within the EUROSPAN consortium to optimally analyze this data48-50.
In summary, women with a traditional lifestyle have a higher cardiovascular health risk compared with women with a modern industrialized lifestyle, possibly because of the following risk factors: higher age, higher consumption of game meat (low in fat, but rich in cholesterol), lower consumption of vegetables (containing β-sitosterol which inhibits cholesterol uptake), less physical activity (especially at leisure), and higher smoking rates. These differences can explain the elevated prevalence of myocardial infarction in TLS women compared with other females. It is proposed that men with a traditional lifestyle have an overall higher orthopedic health risk compared with men with a modern, industrialized lifestyle, possibly because of increased physical activity at work, and higher frequency of work or traffic accidents. Additionally, the substantially lower consumption of dairy products that is also associated with lower bone health (eg bone mineral density) may contribute to the substantially elevated rates of body pain, especially in the back.
Genetics and health - contributions of local populations to global research
Although environmental influences, such as diet and physical activity, are commonly considered important risk factors for various diseases, genetic variants which can be of similar relevance are less present in public awareness. In the NSPHS study, however, an attempt was made to take into account both environmental and genetic effects on health. Due to the complexity of genetic effects it was only possible to deal with this challenge by collaborating with international consortia where studies are pooled to obtain large sample sizes consisting of tens of thousands of individuals. In particular there was cooperation with the European Special Populations Research Network (EUROSPAN, http://www.eurospan.org) which consists of a number of rural populations from Europe (specifically Sweden, Great Britain, The Netherlands, Italy, and Croatia), but also with other international consortia resulting in several high-impact publications on genetic risk factors.
In cooperation with the partners the present authors have identified genes or gene regions with variants that affect, for example, uric acid clearance18, lipid levels19,20 and weight21. These results provide in many cases the first insight into the underlying genetic factors of these traits and disorders. Although most of the genetic risk factors known at present are too weak to have an immediate clinical use, they make it possible to identify metabolic pathways of health relevance and point toward novel potential targets for diagnostic tests and therapeutic interventions. Some of the findings that highlight genetic risk factors for metabolic, cardiovascular, and other disease indicators are now summarized.
Results from EUROSPAN/NSPHS
The body mass index (BMI=weight/height2) is a measure for body fat and an important risk factor for many metabolic and cardiovascular diseases. By joint linkage and genome-wide association analyses, EUROSPAN located a single-nucleotide polymorphism (SNP) close to MGAT1, which is associated with weight21. A genetic region including the GHRHR gene, which affects height22 was also discovered. These findings were later completed by a second gene, the JAZF1 gene, also affecting body size23. Because this gene also has a key function in the metabolism of growth, JAZF1 represents one of the strongest candidates influencing human height identified so far. Sphingolipids have essential roles as structural components of cell membranes and in cell signaling. Disruption of their metabolism causes several diseases, with diverse neurological, psychiatric, and metabolic consequences. The results showed that genetic variants in five genomic regions affect sphingolipids and associate with myocardial infarction19. Creatinine level in the blood serum is an important indicator of kidney function. A genome-wide linkage analysis of serum creatinine in the EUROSPAN cohorts identified a novel locus in the MYH9 gene associating with serum creatinine levels28.
Results from other consortia including EUROSPAN/NSPHS
In a meta-analysis of the CHARGE consortium the role of the FTO and MC4R genes was confirmed and one novel locus identified in the NRXN3 gene influencing central abdominal fat24. As a member of the ENGAGE consortium the present authors found 22 loci associated with blood serum levels of total cholesterol, LDL cholesterol, HDL cholesterol, and triglycerides20. The authors participated in a meta-analysis of 21 studies that identified 16 loci associated with other pre-diabetic traits25. A collaboration of 14 studies identified 9 loci, 5 of which were novel, that affect uric acid levels in the blood serum18. Finally, in the SPIROMETA consortium the authors discovered a number of gene loci affecting various measures of lung function, for example vital capacity27.
Future contributions of local populations to the understanding of complex diseases
Because the mainstream of human health research focuses on urban populations, it might be assumed that local, rural, or remote populations with their specific lifestyles and genetic background might be of limited value for basic research. However, there are several reasons why especially these populations can be a great asset in understanding environmental and genetic risk factors for complex diseases, as explained in the introduction. The authors are currently examining the potential of modeling environmental effects in genome-wide association studies on blood lipid levels to strengthen genetic signals and find novel genetic variants. Initial results show that by adjusting for the environmental effect (ie diet, physical activity) additional genetic factors affecting blood lipid levels can be identified26.
Even if the methodological advantages of studies in rural populations are not harnessed, studies of local populations, such as NSPHS, can still make important contributions to basic research, if they are organized in larger consortia, such as the European Special Populations Research Network (EUROSPAN). These organizations can provide important support to leverage rural studies for participation in large international preclinical and clinical trials. Through this collaboration they may also gain access to resources (eg medical staff, laboratory devices) that will enable population-based health surveys and the identification of specific medical needs of rural or local populations.
Conclusion
In conclusion, there are two points to be highlighted. First, cardiovascular and orthopedic health problems related to sex and lifestyle have been observed in a Northern Swedish population living north of the Arctic Circle. These findings clearly demonstrate the need of rural populations for health assessment and health care. Second, it was found that rural populations feature several advantages for studying the epidemiology of environmental and genetic factors and are suitable participants for large international studies. Studies on environmental and genetic risk factors in rural populations offer great opportunities to better understand the risks for common diseases.
Acknowledgements
The Northern Swedish Population Health Study (NSPHS) was funded by the Swedish Medical Research Council (project number K2007-66X-20270-01-3), the Foundation for Strategic Research (SSF), and the Linneaus Centre for Bioinformatics (LCB). The NSPHS as part of EUROSPAN (European Special Populations Research Network) was also supported by European Commission FP6 STRP grant number 01947 (LSHG-CT-2006-01947). The computations were performed on UPPMAX (http://www.uppmax.uu.se) resources under project p2008027. The authors are grateful for the contribution of district nurse Svea Hennix for data collection and Inger Jonasson for logistics and coordination of the health survey. Finally, the authors thank all the community participants for their interest and willingness to contribute to the study.
References
1. Wakerman J, Humphreys JS, Wells R, Kuipers P, Entwistle P, Jones J. Primary health care delivery models in rural and remote Australia: a systematic review. BMC Health Services Research 2008; 8: 276.
2. Sametinget. The Sami - an Indigenous People in Sweden. (Online) 2005. Available: http://www.regeringen.se/sb/d/5096/a/39702 (Accessed 21 April 2010).
3. Ross A, Johansson Å, Vavruch-Nilsson V, Hassler S, Sjölander P, Edin-Liljegren A et al. Adherence to a traditional lifestyle affects food and nutrient intake among modern Swedish Sami. International Journal of Circumpolar Health 2009; 68(4): 313-416.
4. Ross AB, Johansson A, Ingman M, Gyllensten U. Lifestyle, genetics, and disease in Sami. Croatian Medical Journal 2006; 47(4): 553-565.
5. Ford ES, Giles WH, Mokdad AH. Increasing prevalence of the metabolic syndrome among U.S. Adults. Diabetes Care 2004; 27(10): 2444-2449.
6. Thornton H. The UK Biobank project: Trust and altruism are alive and well: A model for achieving public support for research using personal data. International Journal of Surgery 2009; 7(6): 501-502.
7. Manolio TA, Collins FS. The HapMap and genome-wide association studies in diagnosis and therapy. Annual Review of Medicine 2009; 60: 443-456.
8. Kristiansson K, Naukkarinen J, Peltonen L. Isolated populations and complex disease gene identification. Genome Biology 2008; 9(8): 109.
9. Heutink P, Oostra BA. Gene finding in genetically isolated populations. Human Molecular Genetics 2002; 11(20): 507-2515.
10. Laitinen T. The value of isolated populations in genetic studies of allergic diseases. Current Opinions in Allergy and Clinical Immunology 2002; 2(5): 379-382.
11. Shifman S, Darvasi A. The value of isolated populations. Nature Genetics 2001; 28(4): 309-310.
12. Wright AF, Carothers AD, Pirastu M. Population choice in mapping genes for complex diseases. Nature Genetics 1999; 23(4): 397-404.
13. Rothman KJ, Greenland S, Lash TL. Modern epidemiology 3rd edn. Philadelphia, PA: Lippincott Williams & Wilkins, 2008.
14. Das M, Pal S, Ghosh A. Rural urban differences of cardiovascular disease risk factors in adult Asian Indians. American Journal Human Biology 2008; 20(4): 440-445.
15. Johansson A, Vavruch-Nilsson V, Edin-Liljegren A, Sjölander P, Gyllensten U. Linkage disequilibrium between microsatellite markers in the Swedish Sami relative to a worldwide selection of populations. Human Genetics 2005; 116(1-2): 105-113.
16. Terwilliger JD, Zöllner S, Laan M, Pääbo S. Mapping genes through the use of linkage disequilibrium generated by genetic drift: 'drift mapping' in small populations with no demographic expansion. Human Heredity 1998; 48(3): 138-154.
17. Janssens K, Van Hul W. Molecular genetics of too much bone. Human Molecular Genetics 2002; 11(20): 2385-2393.
18. Kolz M, Johnson T, Sanna S, Teumer A, Vitart V, Perola M et al. Meta-analysis of 28,141 individuals identifies common variants within five new loci that influence uric acid concentrations. PLoS Genetics 2009; 5(6): e1000504.
19. Hicks AA, Pramstaller PP, Johansson A, Vitart V, Rudan I, Ugocsai P et al. Genetic determinants of circulating sphingolipid concentrations in European populations. PLoS Genetics 2009; 5(10): e1000672.
20. Aulchenko YS, Ripatti S, Lindqvist I, Boomsma D, Heid IM, Pramstaller PP et al. Loci influencing lipid levels and coronary heart disease risk in 16 European population cohorts. Nature Genetics 2009; 41(1): 47-55.
21. Johansson A, Marroni F, Hayward C, Franklin CS, Kirichenko AV, Jonasson I et al. Linkage and Genome-wide Association Analysis of Obesity-related Phenotypes: Association of Weight With the MGAT1 Gene. Obesity (Silver Spring) 2010; 18(4): 803-808.
22. Johansson A, Jonasson I, Gyllensten U. Extended haplotypes in the growth hormone releasing hormone receptor gene (GHRHR) are associated with normal variation in height. PLoS ONE 2009; 4(2): e4464.
23. Johansson A, Marroni F, Hayward C, Franklin CS, Kirichenko AV, Jonasson I et al. Common variants in the JAZF1 gene associated with height identified by linkage and genome-wide association analysis. Human Molecular Genetics 2009; 18(2): 373-380.
24. Heard-Costa NL, Zillikens MC, Monda KL, Johansson A, Harris TB, Fu M et al. NRXN3 is a novel locus for waist circumference: a genome-wide association study from the CHARGE Consortium. PLoS Genetics 2009; 5(6): e1000539.
25. Dupuis J, Langenberg C, Prokopenko I, Saxena R, Soranzo N, Jackson AU et al. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nature Genetics 2010; 42(2): 105-116.
26. Igl W, Johansson Å, Wilson JF, Wild SH, Pola?ek O, Hayward C et al. Modeling of environmental effects in genome-wide association studies identifies SLC2A2 and HP as novel loci influencing serum cholesterol levels. PLoS Genetics 2010; 6(1): e1000798.
27. Repapi E, Sayers I, Wain LV, Burton PR, Johnson T, Obeidat M,et al. Genome-wide association study identifies five loci associated with lung function. Nature Genetics 2010; 42(1): 36-44.
28. Pattaro C, Aulchenko YS, Isaacs A, Vitart V, Hayward C, Franklin CS et al. Genome-wide linkage analysis of serum creatinine in three isolated European populations. Kidney International 2009; 76(3): 297-306.
29. World Medical Association (WMA). World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. (Online) 2000. Available: http://www.wma.net/en/30publications/10policies/b3/17c.pdf (Accessed 22 April 2010).
30. Mascalzoni D, Janssens ACJW, Stewart A, Pramstaller P, Gyllensten U, Rudan I et al. Comparison of participant information and informed consent forms of five European studies in genetic isolated populations. European Journal of Human Genetics 2010; 18(3): 296-302.
31. Hassler S, Sjölander P, Ericsson A. Construction of a database on health and living conditions of the Swedish Sami population. Umeå: Umeå University, 2004; 107-124.
32. Weinehall L. Partnership for health. On the role of primary health care in a community intervention programme. Umeå: Department of Public Health and Clinical Medicine, Umeå University, 1997.
33. Weinehall L, Hellsten G, Boman K, Hallmans G, Asplund K, Wall S. Can a sustainable community intervention reduce the health gap?--10-year evaluation of a Swedish community intervention program for the prevention of cardiovascular disease. Scandinavian Journal of Public Health 2001; 56: 59-68.
34. National Food Administration of Sweden. The National Food Administration's food database, version 2009-01-23. (Online) 2009. Available: http://www.slv.se/en-gb/Group1/Food-and-Nutrition/The-Food-Database/ (Accessed 21 April 2010).
35. Liebisch G, Drobnik W, Reil M, Trumbach B, Arnecke R, Olgemoller B et al. Quantitative measurement of different ceramide species from crude cellular extracts by electrospray ionization tandem mass spectrometry (ESI-MS/MS). Journal of Lipid Research 1999; 40(8): 1539-1546.
36. Liebisch G, Drobnik W, Lieser B, Schmitz G. High-throughput quantification of lysophosphatidylcholine by electrospray ionization tandem mass spectrometry. Clinical Chemistry 2002; 48(12): 2217-2224.
37. Liebisch G, Lieser B, Rathenberg J, Drobnik W, Schmitz G. High-throughput quantification of phosphatidylcholine and sphingomyelin by electrospray ionization tandem mass spectrometry coupled with isotope correction algorithm. Biochimica et Biophysica Acta 2004; 1686(1-2): 108-117.
38. Cohen J. Statistical power analysis for the behavioral sciences, 2nd edn. Hillsdale, NJ: Erlbaum, 1988.
39. Altman DG, Bland JM. Statistics notes: absence of evidence is not evidence of absence. BMJ 1995; 311(7003): 485.
40. Proschan MA, Waclawiw MA. Practical guidelines for multiplicity adjustment in clinical trials. Controlled Clinical Trials 2000; 21(6): 527-539.
41. Thompson EA, Shaw RG. Pedigree analysis for quantitative traits: variance components without matrix inversion. Biometrics 1990; 46(2): 99-413.
42. R Development Core Team. R: A Language and Environment for Statistical Computing. (Online) 2009. Available: http://www.R-project.org (Accessed 21 April 2010).
43. National Institutes of Health (NIH). Third Report of the Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (ATP III Final Report). (Online) 2002. Available: http://www.nhlbi.nih.gov/guidelines/cholesterol/atp3full.pdf (Accessed 7 October 2009).
44. Jones PJ, MacDougall DE, Ntanios F, Vanstone CA. Dietary phytosterols as cholesterol-lowering agents in humans. Canadian Journal of Physiology and Pharmacology 1997; 75(3): 217-227.
45. Escurriol V, Cofán M, Moreno-Iribas C, Larrañaga N, Martínez C, Navarro C et al. Phytosterol plasma concentrations and coronary heart disease in the prospective Spanish EPIC cohort. Journal of Lipid Research 2010; 51(3): 618-624.
46. Sjölander P, Hassler S, Janlert U. Stroke and acute myocardial infarction in the Swedish Sami population: incidence and mortality in relation to income and level of education. Scandinavian Journal of Public Health 2008; 36(1): 84-91.
47. Heaney RP. Dairy and bone health. Journal of the American College of Nutrition 2009; 28(Suppl1): 82S-90S.
48. Aulchenko Y, de Koning D, Haley C. Genomewide rapid association using mixed model and regression: a fast and simple method for genomewide pedigree-based quantitative trait loci association analysis. Genetics 2007; 177(1): 577-85.
49. Amin N, van Duijn C, Aulchenko Y. A genomic background based method for association analysis in related individuals. PLoS ONE 2007; 2(12): e1274.
50. Aulchenko Y, Ripke S, Isaacs A, van Duijn C. GenABEL: an R library for genome-wide association analysis. Bioinformatics 2007; 23(10): 1294-6.
51. Leijon M. Hälsan ska kartläggas. Norrbottens-Kuriren. (Online) 2009. Available: http://www.kuriren.nu/nyheter/artikel.aspx?ArticleId=4492247 (Accessed 12 January 2009).
___________________________
Appendix I: Supporting documentation51


