Introduction
A geographic disparity in stroke incidence and mortality exists in many countries and world regions. In Japan, the north-east prefectures have higher stroke mortalities than other parts of the country1. In the USA, stroke incidence and mortality are highest in some south-eastern states, referred to as the 'stroke belt'2. In the UK and Finland, the northern parts of both countries have a higher incidence of stroke than in the south3,4. In Europe, northern countries have a higher stroke incidence than those in the south5.
Explanations have been proposed for this geographic disparity, for example the concentration, in some areas of higher stroke incidence, of traditional risk factors such as hypertension, hypercholesterolemia, higher age, and black African genetic heritage. It is also known that socioeconomic factors such as income level and academic background affect the risk of stroke6 and can contribute to the geographic disparity. However, it has also been reported that individual-level risk factors can only partially explain the disparity of stroke incidence and/or mortality among communities3,7-12.
The community-level geographic and demographic features may be helpful to explain disparity in stroke incidence/mortality. Rural residents are known to be more vulnerable to stroke than their urban counterparts13,14. Nishi et al. reported that Japanese women living in communities with smaller population have a higher stroke mortality than those in other communities, and this was independent of individual-level risk factors, such as age, blood pressure and cholesterol level12. Rural residents have a higher stroke incidence or mortality, even after adjustment for age and sex, in the USA15, Canada16, China17, Bulgaria18, and Potugal19. While higher stroke mortality may be due to a lower availability of stroke treatments, and lower access to medical facilities in rural areas13,20, the higher incidence of stroke cannot, however, be explained by rural treatment or access problems.
Variations in the weather is another community-level variable with the potential to contribute to a geographic disparity of stroke incidence. In several countries stroke mortality and/or incidence reportedly increases in cold months, and thus exposure to low temperatures is a possible risk factor for stroke21-24. Because in Japan, as in many countries in the northern hemisphere, stroke incidence/mortality is higher in northern areas1,3-5, low temperatures may contribite to the north-south disparity. It has also been reported that women in communities with lower temperatures or a higher rainfall had a higher incidence of stroke than women in other communities, independent of individual-level risk factors25.
The geographic disparity of stroke incidence may also be due to an interaction of individual-level and community-level risk factors. To investigate this, it was deemed useful to evaluate the effect of communities' geographic/demographic features on stroke in residents, while accounting for the confounding effects of conventional individual-level risk factors and suspected community-level risk factors, such as meteorological factors. At the same time, the suspected effects of meteorological factors on stroke should also be assessed to exclude the confounding effects of geographic/demographic factors.
Consequently, a cohort study based in 12 communities throughout Japan assessed whether living in a rural community predicted the first-ever-in-a-lifetime stroke incidence of residents, independently of individual-level risk factors and weather conditions.
Methods
Study population
The JMS Cohort Study commenced in 1992. Its primary objective was to clarify the relationship between potential risk factors and cardiovascular diseases in 12 rural municipalities (towns, villages and cities selected from the then 3238 municipalities) in Japan26. Baseline data for the present cohort study were obtained for the period between April 1992 and July 1995 from data collected for a national mass-screening program. Eligible subjects for the mass-screening were residents aged 40-69 years in 11 communities, and residents aged over 30 years in one community. Local government offices in each community issued invitations to eligible residents and 12 490 subjects (4913 males and 7577 females) participated in the study. Among participants, 112 had a past history of stroke and were excluded, leaving 12 378 (4849 males and 7529 females) for inclusion. The total population for each community ranged from 430 to 19 227 (average, 6975). The participation rates for those invited to the mass screening in each community were as follows: 77% in Iwaizumi, 65% in Tako, 78% in Yamato, 69% in Kuze, 90% in Takasu, 79% in Wara, 88% in Sakuma, 30% in Hokudan, 40% in Sakugi, 66% in Okawa, 44% in Ainoshima, and 26% in Akaike27. The overall participation rate was 65.4%. Written informed consent to participate in the study was obtained individually from all mass screening respondents.
Measurement of baseline variables
Body weight was recorded with the subject clothed, and 0.5 kg in summer or 1 kg in other seasons was subtracted from the recorded weight. Body mass index (BMI) was presented as kg/m2. 'Hypertensive subjects' were defined as those with currently-treated hypertension (systolic blood pressure ≥140 mmHg, or diastolic blood pressure ≥90 mmHg). Blood samples were obtained from all participants, and 5532 (44.3%) of these followed overnight fasting. 'Diabetic subjects' were defined as those with currently-treated diabetes, plasma glucose ≥126 mg/dL after overnight fasting, or casual blood glucose ≥200 mg/dL. Age at graduation from the final school attended was used as a proxy measure of socioeconomic status.
Meteorological variables
Weather information was obtained for each community from the nearest observatory of the Japan Meteorological Agency, Ministry of Land, Infrastructure, Transport and Tourism. The distance between the center of a community and its observatory ranged from 0 to 28 km (average, 11.3 km). Meteorological variables used were annual cumulative rainfall (mm) and mean daily temperature for 1 year (°C). All data were derived from the Agency's web site28. The data for each variable were obtained for every year between 1995 and 2005, and the average value of the 11 years was used for analysis. Rainfall and temperature were measured according to 0.5 mm and 0.1°C, respectively.
Geographic and demographic variables
For each community, the population data regarding population size, the 'elderly rate' (those aged ≥65 years) and population density; and the area data of latitude, longitude and altitude, were extracted from Social and Demographic Statistics: the Whole Nation Municipality-level Area Data (Sinfonica, Tokyo, Japan), which was compiled from a number of national censuses. The population and elderly population data used in the present study was the average of that collected in the census years of 1990, 1995 and 2000. Due to massive mergers of municipalities that began in 2004, population data for 2005 was not available for some communities, and therefore it was not used at all. Population density was calculated as persons per km[2]. The elderly rate was presented as the percentage of elderly people in the whole population. The geographic parameters referred to in this article include the area, altitude, latitude and longitude of a community. Demographic parameters indicate population size, density, and the elderly rate.
Follow up
Repeat examinations (part of the national mass-screening program) were used to follow up most subjects on an annual basis. Subjects who did not present for screening examination were contacted by mail or phone. Those examined were asked whether they had experienced a stroke since enrolling, and all who answered in the affirmative were contacted by the present investigators. Any required information was obtained from subjects by visiting public health nurses. The treating hospital records of those with a history of stroke were checked to determine if these subjects were hospitalized for any reason. If a stroke-related incident was suspected, pertinent CT and/or MRI images were obtained for diagnostic confirmation of stroke.
Diagnostic criteria
Diagnosis was determined independently, by means of a diagnosis committee composed of a radiologist, a neurologist and two cardiologists. A diagnosis of stroke was determined on the basis of the presence of a focal and non-convulsive neurological deficit, of clear onset, lasting for 24 hours or longer. Stroke subtypes were confirmed based on CT and/or MRI imaging in all cases except for two (1.1%), whose images were unavailable (in those cases the diagnosis was based on local hospital medical records only). The subtype classification was conducted according to the criteria of the National Institute of Neurological Disorders and Stroke29.
Statistical analysis
Statistical analyses were carried out using SPSS for Windows, v 11.5 (SPSS Inc; Chicago, IL, USA). Continuous variables were compared among communities using ANOVA. Categorical variables were compared using the χ2 test.
Because the data consisted of individual-level data nested within community-level data, it formed a multilevel structure30. Multilevel logistic regression analysis was conducted using the statistical package MLwiN v 2.14 (Centre for Multilevel Modelling, University of Bristol, UK). The regression analysis was performed with a random intercept and fixed slopes model. For evaluation of the association between stroke incidence and each of the geographic and demographic parameters, three separate models were generated for each sex: Model 1 was adjusted for age; Model 2 was adjusted further for BMI, current smoking status, total cholesterol, hypertension, diabetes and final graduation age; and Model 3 was adjusted even further for temperature and rainfall. The results were expressed as odds ratios (OR) and 95% confidence intervals (CI).
Conversely, for evaluating the influence of geographic/demographic factors on weather-stroke association, a similar multilevel regression analysis was conducted and the ORs of temperature (per 1°C) and annual rainfall (per 1000 mm) were calculated, adjusted for all individual-level risk factors and a geographic/demographic factor, which was significantly associated with stroke incidence in Model 1, 2 or 3 in the above analysis.
A second-order, penalized, quasi-likelihood procedure was used to estimate the multilevel regression coefficients. Variance of the intercept in the two-level null random intercept model without any explanatory variable was recognized as the between-area variance. In all statistical tests p<0.05 was considered significant.
Ethical approval
The study design and procedure were approved by the government of each community and the Ethical Committee of Epidemiologic Research at Jichi Medical University, Japan.
Results
Among the eligible study subjects, 95 declined follow up, and seven could not be followed up, providing a participant total of 12 276 (4807 men and 7469 women), 99.2%. Participants' mean age at baseline survey was 55.2 years for men and 55.3 years for the women. Mean follow-up duration was 10.7 years.
The individual-level and community-level variables for each community are shown (Table 1). For all individual-level variables, significant differences were observed among the communities. The stroke incidence among communities was between 1.3 and 6.2 per 1000 person-years. The between-area variance for total incidence of stroke was 0.180 (95% CI: -0.031-0.392) in men, and 0.305 (-0.002-0.632) in women; the variance of cerebral infarction was 0.233 (-0.043-0.509) in men and 0.346 (-0.060-0.752) in women; and the variance of cerebral hemorrhage was 0.113 (-0.175-0.401) in men, and 0.567 (-0.221-1.355) in women.
Associations between geographic/demographic parameters and total stroke incidence are shown for men and women (Table 2). In men, area was associated with increased risk of total stroke in Model 1, but there was no statistical significance in Model 2 or Model 3. In women, low population, low population density, and high altitude increased the risk of stroke both in Model 1 and Model 2. However, in Model 3, where explanatory variables were adjusted for meteorological parameters, there was no statistical significance for population, population density or altitude.
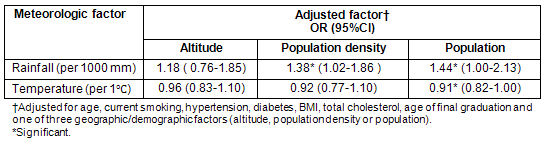
Conversely, the association between each meteorological parameter and stroke in women was significant, even after adjustment for all individual-level risk factors and each of the three geographic/demographic factors (altitude, population density and population) (Table 3). This indicates that the weather-stroke association is stronger than the geography/demography-stroke association. The exception is the association of rainfall when adjusted for individual-level risk factors and altitude, which was not significant.
Relationships between geographic/demographic factors and cerebral infarction are shown (Table 4). In men, no factor was positively or negatively attributed to the incidence of cerebral infarction. In women, low population, low population density and high altitude were associated with an increased risk of cerebral infarction in Model 1, but there was no significance for population or population density in Model 2. Altitude was not significant in Model 3.
Table 5 shows the association of each meteorological factor with cerebral infarction when adjusted for each geographic/demographic factor. When adjusted for altitude, the association between meteorological factors and cerebral infarction was not significant, but when adjusted for population, the association was significant. When adjusted for population density, only the association of rainfall was significant.
The association of each geographic/demographic factor with cerebral hemorrhage was evaluated (not shown in tables). In both men and women, high latitude was associated with increased risk in Model 1 (OR 1.19, 95% CI: 1.01-1.40 in men; OR 1.30, 95% CI: 1.02-1.67 in women), but not in Model 2 or Model 3.
Table 1: Characteristics of participating communities
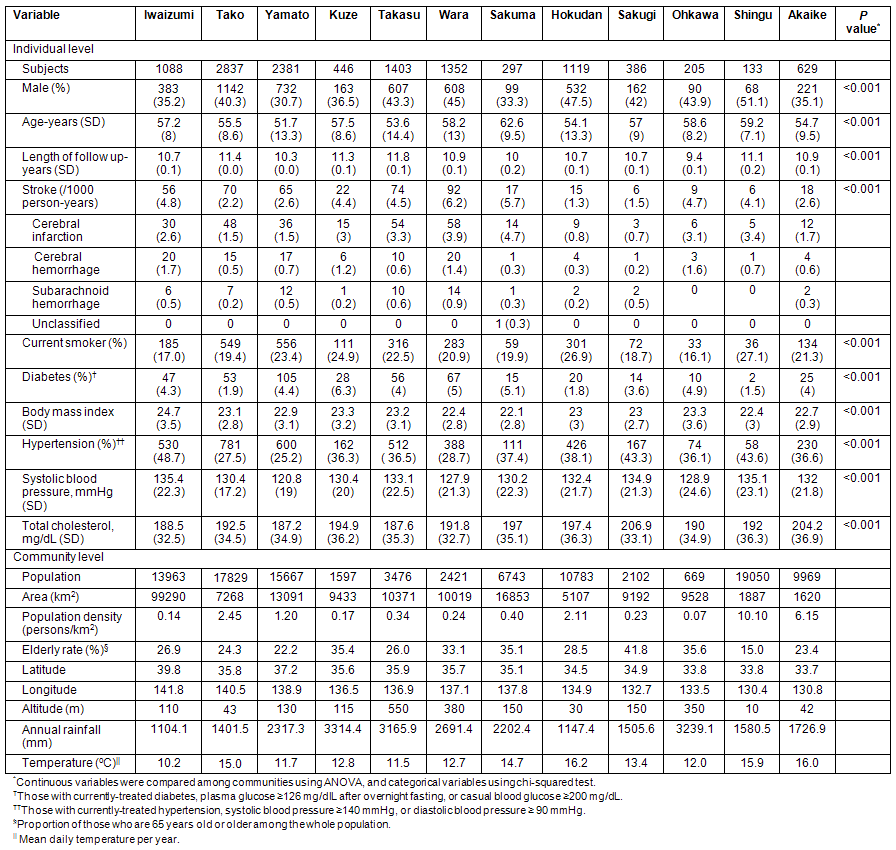
Table 2: Association of each geographic/demographic factor with stroke incidence in men and women
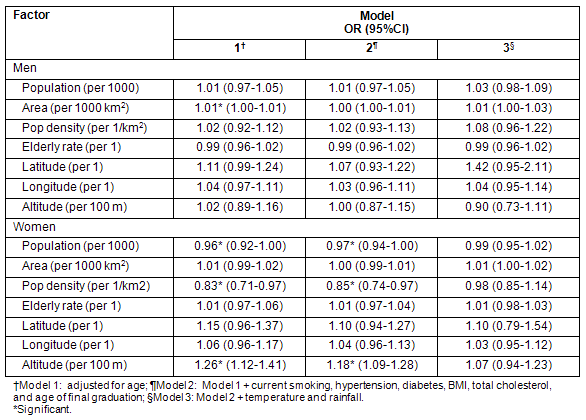
Table 3: Association of each meteorologic factor with stroke incidence in women adjusted for individual-level and geographic/demographic factor
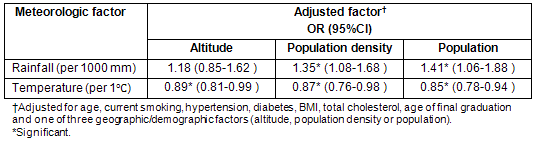
Table 4: Association of each geographic/demographic factor with cerebral infarction incidence in men and women
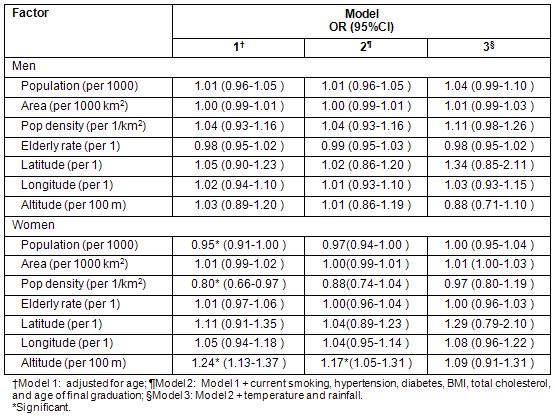
Table 5: Association of each meteorologic factor with incidence of cerebral infarction in women adjusted for individual-level and geographic/demographic factors
Discussion
Women who lived in communities with smaller populations or a lower population density had a higher incidence of stroke, and this was independent of individual-level risk factors such as age, hypertension and diabetes. There was no significant association, however, when adjustment was made for meteorological parameters. Conversely, the association of meteorological parameters with stroke remained significant for individual-level factors and geographic/demographic parameters of the community, even after adjustment.
There was variation among communities in the incidence and mortality of stroke. In men, age-adjusted stroke mortality per 100 000 population was highest at 84.0 in Aomori prefecture, and lowest at 49.6 in Wakayama and Nara prefectures (relative gap between highest and lowest values: 1.7). In women, the highest was in Tochigi prefecture (46.4) and the lowest in Okinawa prefecture (23.1) (relative gap: 2.0). The geographic gap for stroke mortality was larger than that of other major causes of death, such as heart diseases, malignant neoplasms and pneumonia31.
As a potential cause of the geographic disparity in stroke incidence or mortality, the demographic characteristics of a community, such as rurality, are increasingly of interest to researchers. A study from Japan revealed that women residing in municipalities with a population of less than 30 000 had a higher risk of stroke death (odds ratio 1.68), compared with women in municipalities with a population of more than 300 000, even after adjustment for traditional risk factors (eg age, BMI, cholesterol, diabetes, hypertension, smoking status and alcohol drinking)12. Because the present study was not based on incidence data, it is not clear whether the higher mortality in rural areas was due to a higher stroke incidence or a lower survival rate of stroke patients. The present study showed similar results for stroke incidence, suggesting that the previously reported higher stroke mortality for rural women was derived, at least partially, from their higher incidence of stroke. That is, living in a rural area may increase the risk of stroke in women. Another Japanese study showed that the age-adjusted incidence of stroke in a rural community has been consistently higher than in an urban community since 196432.
In the present study, the association between living in a rural community and stroke was independent of traditional risk factors. However, previous studies have not accounted for the confounding effect of potential community-level risk factors, such as weather. This study showed that the link between living in a rural area and stroke in women could be explained by the link between weather and stroke; while the link between weather and stroke was robust against the influence of rurality. Thus, the geographic disparity of stroke incidence in women may be explained by the difference in weather conditions among communities, rather than by urban-rural residence differences. No plausible underlying mechanism has been identified for the association between rural living and stroke. However, low temperature is known to cause an increase in coagulation-related factors such as fibrinogen and factor VII33, an elevation in blood pressure34-36, an exacerbation of hemoconcentration37,38, and an increase in plasma lipids39, which can cause thromboembolic disease, including stroke.
In this and a previous study it was reported that, in terms of stroke incidence, women were more vulnerable to meteorological factors than men25. A possible explanation for this sex difference may be women's greater vascular reaction to cold exposure, due to estrogen-induced increased adrenergic alpha 2C-receptor activity40-43. However, because most of the female stroke cases in our study were post-menopausal, with a consequent low level of blood estrogen, the reasons for the sex difference are unclear.
A limitation of this study is the small number of communities12. This, and the rural-bias of those communities, makes it difficult to generalize the study results to other areas of Japan. In addition, the limited number of communities decreases the power to detect significant difference among them. Indeed, the absence of a significant association between geographic/demographic variables and stroke incidence in Model 3 may be explained by the limited statistical power. Another limitation is that the weather-stroke association seen in this study may be confounded by unmeasured community-level variables (such as local industries types). These issues can be clarified in future studies involving a larger number of communities.
As a geographic parameter, altitude was significantly associated with stroke incidence in women. The association was absent in Model 3 and, therefore, it may be confounded by weather conditions. The associations between weather parameters and stroke were not present when adjusted for altitude. So, as was expected, altitude and weather conditions are likely to have strong correlations.
Acknowledgments
This study was supported by a Scientific Research Grant from the Ministry of Education, Culture, Sports, Science and Technology, Tokyo, Japan, and grants from the Foundation for the Development of the Community, Tochigi, Japan.
References
1. Health and Welfare Statistics Association. Kokumin eisei no doukou [National health trends]. Tokyo: HWSA, 2008. (In Japanese)
2. Gillum RF, Ingram DD. Relation between residence in the southeast region of the United States and stroke incidence. The NHANES I Epidemiologic Followup Study. American Journal of Epidemiology 1996; 144: 665-673.
3. Morris RW, Whincup PH, Emberson JR, Lampe FC, Walker M, Shaper AG. North-south gradients in Britain for stroke and CHD: are they explained by the same factors? Stroke 2003; 34: 2604-2609.
4. Havulinna AS, Paakkonen R, Karvonen M, Salomaa V. Geographic patterns of incidence of ischemic stroke and acute myocardial infarction in Finland during 1991-2003. Annals of Epidemiology 2008; 18: 206-213.
5. Bejot Y, Benatru I, Rouaud O, Fromont A, Besancenot JP, Moreau T et al. Epidemiology of stroke in Europe: geographic and environmental differences. Journal of the Neurological Sciences 2007; 262: 85-88.
6. Avendano M, Kawachi I, Van Lenthe F, Boshuizen, HC, Mackenbach JP, Van den Bos GA et al. Socioeconomic status and stroke incidence in the US elderly: the role of risk factors in the EPESE study. Stroke 2006; 37: 1368-1373.
7. El-Saed A, Kuller LH, Newman AB, Lopez O, Costantino J, McTigue K et al. Geographic variations in stroke incidence and mortality among older populations in four US communities. Stroke 2006; 37: 1975-1979.
8. Voeks JH, McClure LA, Go RC, Prineas RJ, Cushman M, Kissela BM et al. Regional differences in diabetes as a possible contributor to the geographic disparity in stroke mortality: the Reasons for Geographic and Racial Differences in Stroke Study. Stroke 2008; 39: 1675-1680.
9. El-Saed A, Kuller LH, Newman AB, Lopez O, Costantino J, McTigue K et al. Factors associated with geographic variations in stroke incidence among older populations in four US communities. Stroke 2006; 37: 1980-1985.
10. Howard G, Prineas R, Moy C, Cushman M, Kellum M, Temple E et al. Racial and geographic differences in awareness, treatment, and control of hypertension: the Reasons for Geographic and Racial Differences in Stroke Study. Stroke 2006; 37: 1171-1178.
11. Yang D, Howard G, Coffey CS, Roseman J. The confounding of race and geography: how much of the excess stroke mortality among African Americans is explained by geography? Neuroepidemiology 2004; 23: 118-122.
12. Nishi N, Sugiyama H, Kasagi F, Kodama K, Hayakawa T, Ueda K et al. Urban-rural difference in stroke mortality from a 19-year cohort study of the Japanese general population: NIPPON DATA80. Social Science & Medicine 2007; 65: 822-832.
13. Joubert J, Prentice LF, Moulin T, Liaw ST, Joubert LB, Preux PM et al. Stroke in rural areas and small communities. Stroke 2008; 39: 1920-1928.
14. Pearson TA, Lewis C. Rural epidemiology: insights from a rural population laboratory. American Journal of Epidemiology 1998; 148: 949-957.
15. Adams PF, Hendershot GE, Marano MA. Current estimates from the National Health Interview Survey, 1996. Vital & Health Statistics 1999; 200: 1-203.
16. Yiannakoulias N, Svenson LW, Hill MD, Schopflocher DP, Rowe BH, James RC et al. Incident cerebrovascular disease in rural and urban Alberta. Cerebrovascular Diseases 2004; 17: 72-78.
17. Zhang XH, Guan T, Mao J, Liu L. Disparity and its time trends in stroke mortality between urban and rural populations in China 1987 to 2001: changing patterns and their implications for public health policy. Stroke 2007; 38: 3139-3144.
18. Powles J, Kirov P, Feschieva N, Stanoev M, Atanasova V. Stroke in urban and rural populations in north-east Bulgaria: incidence and case fatality findings from a 'hot pursuit' study. BMC Public Health 2002; 2: 24.
19. Correia M, Silva MR, Matos I, Magalhaes R, Lopes JC, Ferro JM et al. Prospective community-based study of stroke in Northern Portugal: incidence and case fatality in rural and urban populations. Stroke 2004; 35: 2048-2053.
20. Leira EC, Hess DC, Torner JC, Adams HP Jr. Rural-urban differences in acute stroke management practices: a modifiable disparity. Archives of Neurology 2008; 65: 887-891.
21. The Eurowinter Group. Cold exposure and winter mortality from ischaemic heart disease, cerebrovascular disease, respiratory disease, and all causes in warm and cold regions of Europe. Lancet 1997; 349: 1341-1346.
22. Pan WH, Li LA, Tsai MJ. Temperature extremes and mortality from coronary heart disease and cerebral infarction in elderly Chinese. Lancet 1995; 345: 353-355.
23. Shinkawa A, Ueda K, Hasuo Y, Kiyohara Y, Fujishima M. Seasonal variation in stroke incidence in Hisayama, Japan. Stroke 1990; 21: 1262-1267.
24. Turin TC, Kita Y, Murakami Y, Rumana N, Sugihara H, Morita Y et al. Higher stroke incidence in the spring season regardless of conventional risk factors: Takashima Stroke Registry, Japan, 1988-2001. Stroke 2008; 39: 745-752.
25. Matsumoto M, Ishikawa S, Kajii E. Cumulative effects of weather on stroke incidence: a multi-community cohort study in Japan. Journal of Epidemiology 2010; 20: 136-142.
26. Ishikawa S, Kayaba K, Gotoh T, Nago N, Nakamura Y, Tsutsumi A et al. Incidence of total stroke, stroke subtypes, and myocardial infarction in the Japanese population: the JMS Cohort Study. Journal of Epidemiology 2008; 18: 144-150.
27. Ishikawa S, Gotoh T, Nago N, Kayaba K. The Jichi Medical School (JMS) Cohort Study: design, baseline data and standardized mortality ratios. Journal of Epidemiology 2002; 12: 408-417.
28. Japan Meteorological Agency. Kako no kisyo data kensaku (Meteolorogical database search). Available: http://www.data.jma.go.jp/obd/stats/etrn/index.php (Accessed 17 November 2008). (In Japanese)
29. Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993; 24: 35-41.
30. Twisk JWR. Applied multilevel analysis. Cambridge: Cambridge University Press, 2006.
31. Ministry of Health, Labour and Welfare. Kokumin eisei no doukou 2008 [Status of population health 2008]. Journal of Health and Welfare Statistics 2008; 55(9): 402-403.
32. Kitamura A, Sato S, Kiyama M, Imano H, Iso H, Okada T et al. Trends in the incidence of coronary heart disease and stroke and their risk factors in Japan, 1964 to 2003: the Akita-Osaka study. Journal of the American College of Cardiology 2008; 52: 71-79.
33. Woodhouse PR, Khaw KT, Plummer M, Foley A, Meade TW. Seasonal variations of plasma fibrinogen and factor VII activity in the elderly: winter infections and death from cardiovascular disease. Lancet 1994; 343: 435-439.
34. Imai Y, Munakata M, Tsuji I, Ohkubo T, Satoh H, Yoshino H et al. Seasonal variation in blood pressure in normotensive women studied by home measurements. Clinical Science (London) 1996; 90: 55-60.
35. Woodhouse PR, Khaw KT, Plummer M. Seasonal variation of blood pressure and its relationship to ambient temperature in an elderly population. Journal of Hypertension 1993; 11: 1267-1274.
36. Alpérovitch A, Lacombe JM, Hanon O, Dartigues JF, Ritchie K, Ducimetière P et al. Relationship between blood pressure and outdoor temperature in a large sample of elderly individuals: the Three-City study. Archives of Internal Medicine 2009; 169: 75-80.
37. Neild PJ, Syndercombe-Court D, Keatinge WR, Donaldson GC, Mattock M, Caunce M. Cold-induced increases in erythrocyte count, plasma cholesterol and plasma fibrinogen of elderly people without a comparable rise in protein C or factor X. Clinical Science (London) 1994; 86: 43-48.
38. Keatinge WR, Coleshaw SR, Cotter F, Mattock M, Murphy M, Chelliah R. Increases in platelet and red cell counts, blood viscosity, and arterial pressure during mild surface cooling: factors in mortality from coronary and cerebral thrombosis in winter. BMJ 1984; 289: 1405-1408.
39. Woodhouse PR, Khaw KT, Plummer M. Seasonal variation of serum lipids in an elderly population. Age and Ageing 1993; 22: 273-278.
40. Sato F, Matsushita S, Hyodo K, Akishima S, Imazuru T, Tokunaga C et al. Sex difference in peripheral arterial response to cold exposure. Circulation Journal 2008; 72: 1367-1372.
41. Eid AH, Maiti K, Mitra S, Chotani MA, Flavahan S, Bailey SR et al. Estrogen increases smooth muscle expression of alpha2Cadrenoceptors and cold-induced constriction of cutaneous arteries. American Journal of Physiology - Heart and Circulatory Physiology 2007; 293: H1955-1961.
42. Bartelink ML, De Wit A, Wollersheim H, Theeuwes A, Thien T. Skin vascular reactivity in healthy subjects: influence of hormonal status. Journal of Applied Physiology 1993; 74: 727-732.
43. Cooke JP, Creager MA, Osmundson PJ, Shepherd JT. Sex differences in control of cutaneous blood flow. Circulation 1990; 82: 1607-1615.
Abstract
Introduction: An urban-rural gap in stroke incidence or mortality has been reported. However, whether the effect of rurality on stroke is independent of the distribution of conventional individual-level risk factors and other community-level risk factors is inconclusive.
Methods: A cohort study was conducted involving 4849 men and 7529 women residing in 12 communities throughout Japan. Baseline data were obtained between April 1992 and July 1995. Follow up was conducted annually to capture first-ever-in-life stroke events. During that period, geographic, demographic and weather information was obtained for each community. Multi-level logistic regression analysis was conducted to evaluate the association between stroke incidence and each geographic/demographic factor adjusted for meteorological parameters (temperature and rainfall), in addition to individual-level risk factors (age, body mass index, smoking, total cholesterol, hypertension, and diabetes).
Results: Throughout an average of 10.7 years' follow up, 229 men and 221 women with stroke events were identified. In women, low population (odds ratio [OR] per 1000 persons 0.97; 95% confidence interval 0.94-1.00), low population density (OR per 1/km2 0.85; 0.74-0.97) and high altitude (OR per 100 m 1.18; 1.09-1.28) increased the risk of stroke independently of individual-level risk factors; however, significance was absent for all three associations when further adjusted for weather parameters. Conversely, the association between each meteorological parameter and stroke in women was significant, even after adjustment for each of the three geographic/demographic factors. Similar results were obtained for cerebral infarction.
Conclusion: The association between living in rural communities and stroke may be caused by the confounding effect of weather conditions in the communities studied.
Key words: demography, geography, meteorological factors, stroke.
You might also be interested in:
2008 - Quality of life in patients with sickle cell disease in Jamaica: rural-urban differences
2006 - Comparison of stroke among Christians and Muslims in Thrace, Greece

