Menopause is caused by the depletion of ovarian function in women, followed by the cessation of menstruation. Most women spend one-third to half of their lifetime in postmenopause. The increasing length of postmenopausal life emphasises the importance of the effects of menopause1, which include biological and psychosocial changes.
Menopause may be associated with vasomotor symptoms, bone loss, urogenital atrophy, urinary tract infections and incontinence, increased cardiovascular risk, somatic symptoms, sexual dysfunction and decreased libido. These symptoms may lead to social impairment and work related difficulties that significantly decrease overall quality of life.
Cross-cultural research on menopause suggests that the symptoms of and attitudes to menopause vary considerably according to environment, health status and cultural paradigms around women's health2. Thus, evaluating these aspects may be helpful in gauging the effects of menopausal symptoms. Demographic characteristics, and psychosocial and lifestyle factors are important determinants of postmenopausal symptoms.
This observational study was designed to: (i) evaluate the prevalence of risk factors for osteoporosis and cardiovascular disease and the prevalence and severity of the appearance of menopausal symptoms among rural and urban Spanish menopausal women; (ii) identify the main factors responsible for severity of symptoms; and (iii) detect symptom differences between rural and urban women.
Subject design and sampling
The FASEM is a Spanish scientific forum that studies menopausal women. The FASEM study is a 2006 cross-sectional study that was conducted throughout Spain on women aged 45-65 years attending general practice clinics.
The present study, part of a set of studies called FASEM, focused on rural-urban differences among the women of the FASEM study. The two-stage sampling was the same for both the larger and the present study. The first stage involved a stratified random sample (for provinces and home environment) of 1401 Spanish GPs, one in seven active Spanish GPs. The overall response rate was 87.1% (1221 GPs in 329 areas in 50 Spanish provinces). The second stage involved a random sample of all women aged 45-65 years who were on the health registers of participating GPs (all women living in his/her area). A systematic sampling technique was used to select every fifth woman. Each GP then personally invited the selected women from his or her practice to take part in the study.
The questionnaire used had been designed and pre-tested specifically for the study, although it was not validated. Informed consent was obtained for every woman who agreed to enter the study.
Women were excluded for the following reasons: gynaecological cancer, bilateral oopherectomy, dementia, organic brain syndrome or Alzheimer's disease, missing data for menopausal status or symptoms, or under 55 years with a history of hysterectomy .
Measurements
The questionnaire contained 21 questions addressing sociodemographic factors, medical history and lifestyle factors. Sociodemographic factors included age, marital status, urban or rural living environment (the majority of its active population was involved in agricultural production or services related to local life), educational level and social level. Marital status was categorised as married, unmarried (woman not living with partner), widowed or divorced or separated. Participants' educational levels were divided into four levels: lower than primary education, primary education (school attendance up to age 10 years), secondary education (school attendance up to age 17 years) and university education. Social level was estimated by assessing financial income and employment status. The scale used was proposed by the Spanish Society of Epidemiology3 and the results were grouped into three categories: low, intermediate and high.
Medical history questions related to: (a) personal and family background - family history of osteoporosis, personal history of fracture, medication use predisposing to osteoporosis (including oral glucocorticoids, anticonvulsants and anti-thyroids) used for at least 90 days, and diseases at risk for osteoporosis (eg chronic hepatopathy, hyperthyroidism and hyperparathyroidism); (b) gynaecological data - gynaecological history and menopausal symptoms; and (c) physical data - BMI, blood pressure, blood glucose and lipid levels.
Body mass index was categorised as normal weight (BMI 18.5-24.9), overweight (BMI 25-29.9), obesity I (BMI 30-34.9), obesity II (BMI 35-39.9) and obesity III (BMI ≥40). A positive family history was recorded when the women had a first degree female relative who had sustained a fracture of the hip or wrist as a result of osteoporosis. The systolic and diastolic blood pressure values used in the analysis as outcomes were averages of the second and third readings (2 min apart). Hypertension was defined as systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥ 90 mmHg or currently on treatment with antihypertensive medication. Hypercholesterolemia was defined as total serum cholesterol ≥230 mg/dL and diabetes as total serum glucose ≥126 mg/dL.
Lifestyle factors were alcohol intake, smoking status, consumption of dairy products and degree of exercise. Tobacco use was categorised as non-smoker, 1-10 cigarettes per day, 11-20 cigarettes per day and >20 cigarettes per day. Alcohol use was categorised as no alcohol, 1-5 drinks per day (moderate drinkers) and >6 drinks per day (heavy drinkers). A diet with poor dairy product content (and therefore low in calcium) was recorded if <500 mL milk per day was consumed, with cheese, yoghurt, ice cream or milk pudding taken once a week or less. Two questions were used to assess exercise on the questionnaire. Moderate exercise was defined as 'exercise or work lasting 30 min or more without stopping'. Weight-bearing exercise was defined as 'exercise where your legs bear your body weight, such as walking, jogging, dancing, or weight training'. Exercise was categorised as never, less than once per week, 1-2 times per week and at least 3 times per week.
Women were classified as peri- or postmenopausal, according to the Straw (Stages of Reproductive Aging Workshop) definitions4. Thus, perimenopause was defined as the time around menopause during which menstrual cycle and endocrine changes are occurring but 12 months of amenorrhea has not yet occurred. Post-menopause begins at the time of the last menstrual period, although it is not recognised until after 12 months of amenorrhea.
A cumulative symptom score, the Kupperman index5, was used to classify the severity and intensity of menopausal symptoms, using 11 dimensions (hot flushes/night sweats, paraesthesia, insomnia/sleep disturbances, depression, irritability, dizziness, tiredness/weakness, arthralgia/myalgia, headache, palpitations and tingling). The scores range from 0 to 3 (none, mild, moderate and severe). Each complaint was scored individually and multiplied by its rate of importance: hot flushes (4), paraesthesia (2), insomnia (2), irritability (2), and all other symptoms (1). The sum of these scores presents an estimation of overall severity from menopause (none: 0-14 points; mild: 15-20; moderate 21-35 and severe: >35).
Statistical analysis
Data were analysed using the SPSS (www.spss.com). Quantitative variables are expressed as mean and standard deviation (SD) and qualitative variables as percentages with 95% confidence intervals (CI). Categorical variables were compared using χ2 test (with Yates correction when necessary) or Fisher's exact test. A logistic regression analysis was carried out with the presence of severity of the menopausal symptoms as a dependent variable and progressive elimination of independent variables (sociodemographic, medical history and lifestyle factors). A p-value <0.05 was considered significant.
Ethics approval
This study was approved by the institutional ethical review committee of the Guadalajara Health Area (#12/05).
The sample was 12 068 women, 168 of whom were excluded according to study criteria, and 1386 declined the invitation to participate. The remaining 10 514 women enrolled in the study were divided into two subgroups: 69.7% urban (7327 women) and 30.3% were rural (3187 women). The mean age of the sample was 57.9 (±7.1) years. Their sociodemographic data are presented according to home location (Table 1).
Table 1: Sample distribution according to age, marital status,
educational level and menopausal status and home location
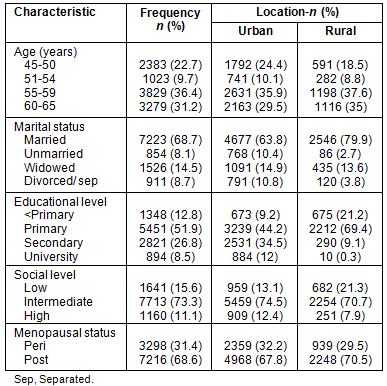
The prevalence of risk factors for cardiovascular disease among the population studied was 74.8% (7864 women). Of these, 3209 (40.8%) presented one risk factor, 2406 (30.6%) two, 1478 (18.8%) three and 771 (9.8%) more than three. Obesity (46.1%), hypercholesterolemia (37.5%) and arterial hypertension (35.5%) were the most common cardiovascular risk factors in the rural population, while in the urban it was obesity (43.5%), arterial hypertension (37.2%) and smoking (33.4%) (Table 2).
Risk factors for osteoporosis were identified in 7107 patients (67.6%), of whom 2754 (38.7%) had one risk factor, 2189 (30.8%) two, 1359 (19.1%) three and 805 (11.3%) more than three. Among risk factors for osteoporosis in the rural women, 46% did no exercise, 43.1% were taking medication that could influence bone metabolism and/or increase risk of fracture and 24.4% had a poor dairy content diet; whereas in the urban women, 56.9% did no exercise, 47.1% were taking at-risk medication and 28.7% smoked (Table 2).
Table 2: Prevalence of risk factors for cardiovascular disease and osteoporosis according to home location
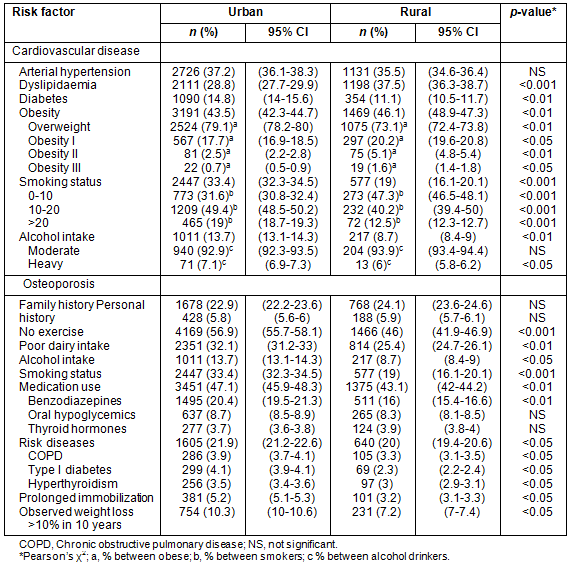
The most common symptoms experienced by urban menopausal women were hot flushes (49.9%), insomnia (46.9%) and irritability (43.4%); whereas in the rural women these were hot flushes (55%), sleeping problems (42.8%) and depressive mood (41.7%) (Table 3). In general, a significant increasing trend in the rate of menopausal symptoms was observed from peri- to postmenopause in both urban and rural women. The prevalence of joint pain and depressive mood was higher in perimenopausal than in postmenopausal women in both groups although it was not statistically significant (Table 3).
Rural women had the highest frequencies of 4 of the 11 symptoms (hot flushes, depressive mood, joint pain and tingling), whereas urban women had the highest frequency of 7 symptoms. The difference was statistically significant in rural women for hot flushes (Table 4).
Symptoms were severe in 3.3% of the sample, moderate in 27.3%, mild in 24.6% and 44.8% had no symptoms. In the rural women symptoms were severe in 2.7% of the sample, moderate in 27.7%, mild in 24.7% and 44.9% had no symptoms, while in the urban environment they were severe in 3.7%, moderate in 27.6%, mild in 24.9% and 43.8% had no symptoms. Rural women had statistically significantly (p<0.01) fewer symptoms. The distribution of menopausal symptom intensity is shown according to age, marital status, home location, educational level and social status (Table 5).
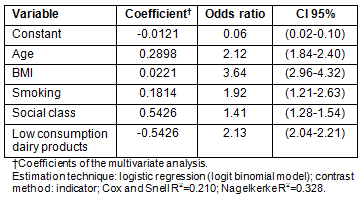
In the logistic regression analysis, the variables age, BMI, smoking, social status and low consumption of dairy products contributed significantly and independently to the severity of menopausal symptoms (Table 6). The variable home location did not influence this severity.
Table 3: Frequency of menopausal symptoms in rural and urban women according to menopause status
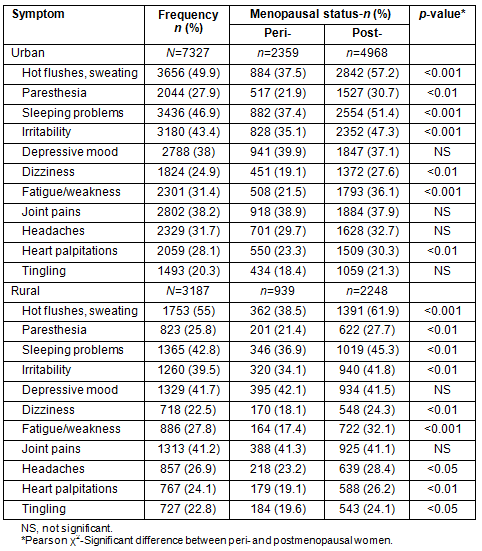
Table 4: Menopausal symptom differences between rural and urban women
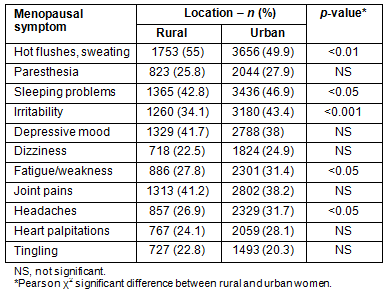
Table 5: Distribution of Kupperman index according to age,
marital status, educational level, social class and home location
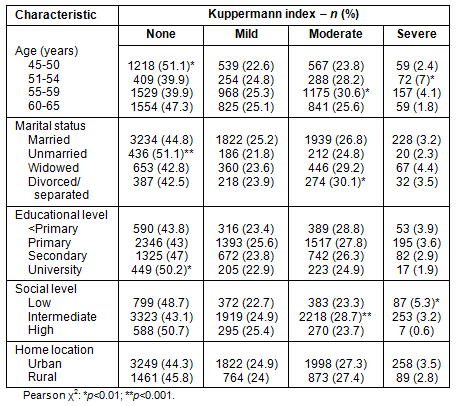
Table 6: Significant variables associated with
the severity of menopausal symptoms
Discussion
This study forms is part of a more extensive work entitled FASEM, which was carried out across Spain and whose general data have already been published6. The authors of this work were concerned with analysing the possible differences between rural and urban peri- and postmenopausal women, bearing in mind that these two populations had different lifestyles, age, marital statuses, educational and economic levels.
This study found statistically significant differences between urban and rural populations regarding some cardiovascular risk factors, the frequency of hot flushes and the severity of menopausal symptoms. The rural sample was an older population, with lower educational and economic levels and a lower proportion of single, widowed or divorced women (Table 1).
Interpretation of the findings in the light of evidence
Cardiovascular risk factors: A high prevalence of cardiovascular risk factors (74.3%) was found throughout the whole sample studied. A higher percentage of cardiovascular risk factors was observed in the urban environment (74.6%) compared with the rural (73.6%). Although the difference was not statistically significant it should be taken into account given that the rural population was of older average age, as other authors have suggested7,8.
The most prevalent risk factor in both communities was obesity, followed by hyperglycaemia and high blood pressure in the rural women, and high blood pressure and smoking in urban women. There was a significant difference in favour of the rural women in obesity and hyperlipemia, and in the urban women in diabetes, smoking and alcohol consumption, while the prevalence of high blood pressure was similar in both. These differences may be due to urban women having more westernised lifestyles9. Overall, these results are consistent with other studies, finding that these are the most prevalent cardiovascular risk factors in menopausal woman10-13.
Cardiovascular disease is considered the major cause of mortality in women from industrial western countries, especially from 50 years onwards, coinciding with the onset of the menopause and the metabolic changes that occur peri- and post-menopause. It is fundamental for physicians caring for menopausal women to advise, diagnose and treat all modifiable risk factors, including diabetes14, with special emphasis on blood pressure, which is considered the most important determinant in morbidity and mortality due to cardiovascular disease in these women15,16.
Risk factors for osteoporosis: It was found that 67.6% of women from the sample had at least one risk factor for osteoporosis (68.1% urban compared with 66.5% rural), although the difference was not significant, and this is consistent with the literature17-19. In both communities the most prevalent risk factor was 'no exercise'. In rural women it was followed by poor dairy intake, while in urban women it was smoking. According to other studies20,21, these are the most frequent osteoporosis risk factors in menopausal women.
It is surprising that 45.9% of women in the sample (47.1% in the urban environment and 43.1% in the rural environment) were taking medication that increased their risk of osteoporosis, and this is a higher percentage than in another study22. With close to one in two of the women studied taking medication that might alter bone metabolism, and not all these being used to treat menopausal symptoms, this might indicate over-treatment of some menopausal women.
During perimenopause, both the quantity and quality of bone reduces, causing an increased risk of bone fracture in postmenopausal women. Although many of the factors associated with osteoporosis fractures are well-known, measures to identify and treat women at risk are not used routinely in clinical practice, leading to a diagnosis of osteoporosis only after a fracture9. It is therefore important to identify menopausal women at risk, especially those who undergo early menopause, who may benefit from early intervention r to maintain or increase their bone mass.
Prevalence of menopausal symptoms: The prevalence of menopausal symptoms in the sample was higher in urban (89.6%) than rural women (87.6%), although not significantly so, in agreement with other studies23. However, other authors in central Spain found the opposite24, although their study population was smaller, closely associated with a specific geographic location and contained a larger number of variables.
In both urban and rural women there were high rates of hot flushes and insomnia; however, the groups differed according to the third most prevalent symptom: irritability in urban and depression in rural women. Compared with urban women, rural women had a higher frequency of hot flushes, depression, joint pain and tingling symptoms. This disparity, especially in the frequency of hot flushes where the difference was statistically different, suggests that home location has an impact. However, it is not easy to explain this with social, economic and demographic diversities because other factors must also play an important role, such as structural differences in the two environments24. The importance of correctly addressing these symptoms is highlighted, for insomnia, tiredness and irritability may affect daily activities, as well as family and social relationships.
Symptom differences between peri- and postmenopausal women: In the main sample, perimenopausal women had a higher (not significant) frequency of two of the listed symptoms (joint pains and depression), while postmenopausal women suffered from the nine remaining symptoms, eight of which were statistically significant. These results were reproduced in the present study, although there was a greater proportion of perimenopausal rural women (not statistically significant) with depression and joint pains.
In line with other studies23,25, it was observed that joint pains were a major problem for perimenopausal women, affecting their quality of life. The present study also found that depression affected perimenopausal women more, which suggests the importance of the period prior to the menopause in its effect on women.
Severity of menopausal symptoms: According to other authors, the factors most related to an increase in suffering from severe menopausal symptoms are smoking, maternal history, a history of premenstrual pain, high body temperature, little physical activity and low socioeconomic status26,27.
Other studies agreed that obesity28,29 and smoking28,30 play an important role in an increased risk of hot flushes; however, a correlation between menopausal symptoms and low socioeconomic status has also been considered31. Unlike some studies32,33, the present study found no statistically significant association between performing physical exercise and alcohol intake with greater severity of menopausal symptoms, consistent with other studies34,35.
Although rural women had significantly less severe menopausal symptoms, rural or urban home location did not contribute significantly or independently to the severity of symptoms.
Strengths and limitations
Regarding the methodology of the study, the authors consider that the sample to be adequately representative of the population visiting their GP in Spain. However, it should be noted that its strength is the lack of a self-selection bias by GPs, guaranteed by the random sampling procedure. Moreover, the selection of GPs was made uniformly and can therefore be regarded as representative of Spain as a whole.
Regarding limitations, as the group of interviewees was large, the classification criteria of some variables may not have been uniformly applied throughout the study. Furthermore, some data may be biased by under-reporting (eg substance abuse such as smoking and alcohol intake), although in such a large sample this had no significant effect on the final results. The Kupperman Index is not validated according to psychometric standards36,37; however, it is still in use in medical practice to monitor menopausal symptoms and is widely accepted. Another limitation is that only the population attending clinical practice was studied, so the sample cannot be considered representative of the entire general population. However, the authors believe this point is nominal as more than 90% of the Spanish population attends clinical practice and therefore the results obtained should be quite similar to those in the general population.
The observational study revealed certain differences between urban and rural population with respect to some cardiovascular risk factors, frequency of hot flushes and severity of menopausal symptoms that may be explained by the complex interaction among biological, ecological and behavioural factors. The results of the present study should be confirmed by future research.
Acknowledgements
The authors express sincere gratitude to all investigators who actively participated in this study. Although they are too many to list, without their dedication and quality of work this publication would not have been possible. Also thanked are Almirall Pharmaceuticals and Sanofi-Aventis for their support, which was essential in order to complete this study.
References
1. Moon-Soo L, Jong-Hun K, Man Sik P, Jaewon Y, Young-Hoon K, Seung-Duk K et al. Factors Influencing the Severity of Menopause Symptoms in Korean Post-menopausal Women. Journal of Korean Medical Science 2010; 25: 758-765.
2. Im EO, Liu Y, Dormire S, Chee W. Menopausal symptom experience: an online forum study. Journal of Advanced Nursing 2008; 62: 541-550.
3. Alvarez C, Alonso J, Domingo A, Regidor E. La medición de la clase social en Ciencias de la Salud. Sociedad Española de Epidemiología. Barcelona: Editores SG, 1995.
4. Soules MR, Sherman S, Parrot E, Rebar R, Santero N, Uyian W et al. Executive summary: stages of reproductive aging workshop (STRAW) Park City, UTA. July 2001. Menopause 2001; 8: 402-407.
5. Kupperman HS, Wetchchler BB, Blat MHG. Contemporary therapy of the menopausal syndrome. JAMA 1959; 171: 1627-1637.
6. Pérez JA, Garcia FC, Palacios S, Pérez M. Epidemiology of risk factors and symptoms associated with menopause in Spanish women. Maturitas 2009; 62: 30-36.
7. Heidari R, Sadeghi M, Talaei M, Rabiei K, Mohammadifard N, Sarrafzadegan N. Metabolic syndrome in menopausal transition: Isfahan Healthy Heart Program, a population based study. Diabetolology and Metabolic Syndrome 2010; 2: 59.
8. Rojas R, Aguilar-Salinas CA, Jiménez-Corona A, Shamah-Levy T, Rauda J, Avila-Burgos L et al. Metabolic syndrome in Mexican adults: results from the National Health and Nutrition Survey 2006. Salud Publica de Mexico 2010; 52(Suppl1): 11-18.
9. Ciaschini PM, Straus SE, Dolovich LR, Goeree RA, Leung KM, Woods CR et al. Community based intervention to optimize osteoporosis management: randomized controlled trial. BMC Geriatrics 2010; 10: 60.
10. Chedraui P, Pérez-López FR, Blümel JE, Hidalgo L, Barriga J. Hyperglycemia in postmenopausal women screened for the metabolic syndrome is associated to increased sexual complaints. Gynecological Endocrinology 2010; 26: 86-92.
11. Bhagat M, Mukherjee S, De P, Goswami R, Pal S, Das M, Ghosh A. Clustering of cardiometabolic risk factors in Asian Indian women: Santiniketan women study. Menopause 2010; 17: 359-364.
12. Romaguera J, Ortiz AP, Roca FJ, Colón G, Suárez E. Factors associated with metabolic syndrome in a sample of women in Puerto Rico. Menopause 2010; 17: 388-392.
13. Petri Nahas EA, Padoani NP, Nahas-Neto J, Orsatti FL, Tardivo AP, Dias R . Metabolic syndrome and its associated risk factors in Brazilian postmenopausal women. Climateric 2009; 12: 431-438.
14. Wenger NK, Mischke JM, Schroeder R, Schroeder K, Collins P, Grady D et al. Electrocardiograms of menopausal women with coronary heart disease or at increased risk for its occurrence. American Journal of Cardiology 2010; 106: 1580-1587.
15. Nuzzo A, Rossi R, Modena MG. Hypertension alone or related to the metabolic syndrome in postmenopausal women. Expert Review of Cardiovascular Therapy 2010; 8: 1541-1548.
16. Taddei S. Blood pressure through aging and menopause. Climateric 2009; 12(Suppl1): 36-40.
17. Rosengren BE, Ahlborg HG, Gärdsell P, Sernbo I, Daly RM, Nilsson JA et al. Bone mineral density and incidence of hip fracture in Swedish urban and rural women 1987-2002. Acta Orthopaedica 2010; 81: 453-459.
18. Vanasse A, Courteau J, Cohen AA, Orzanco MG, Drouin C. Rural-urban disparities in the management and health issues of chronic diseases in Quebec (Canada) in the early 2000s. Rural and Remote Health 2010; 10: 1548. (Online) 2010. Available: www.rrh.org.au (Accessed 15 April 2013).
19. Pinheiro MM, Ciconelli RM, Jacques Nde O, Genaro PS, Martini LA, Ferraz MB. The burden of osteoporosis in Brazil: regional data from fractures in adult men and women--the Brazilian Osteoporosis Study (BRAZOS). Revista Brasileira de Reumatologia 2010; 50: 113-127.
20. Blotman F, Cortet B, Hilliquin P, Avouac B, Allaert FA, Pouchain D et al. Characterisation of patients with postmenopausal osteoporosis in French primary healthcare. Drugs and Aging 2007; 24: 603-614.
21. Chen JS, Hogan C, Lyubomirsky G, Sambrook PN. Management of osteoporosis in primary care in Australia. Osteoporosis International 2009; 20: 491-496.
22. Dumitrescu B, Van Helden S, Ten Broeke R, Nieuwenhuijzen-Kruseman A, Wyers C, Udrea G et al. Evaluation of patients with clinical fracture and osteoporosis, a multidisciplinary approach. BMC Musculoskeletal Disorders 2008; 9: 109-119.
23. Carranza-Lira S, Flores-Miranda MA, Gómez-Brigada I. Comparison of climacteric syndrome symptoms among perimenopausal women from Mexico City and one Zapotec community of Oaxaca, Mexico. Ginecologia y Obstetricia de Mexico 2010; 78: 116-120.
24. Bernis C, Sven Reher D. Environmental contexts of menopause in Spain: comparative results from recent research. Menopause 2007; 4: 777-787.
25. Alexander JL, Dennerstein L, Woody NF. Arthralgias, bodily aches and pains and somatic complaints in midlife women: etiology, pathophysiology and differential diagnosis. Expert Review Neurotherapeutics 2007; 7(11 Suppl): S15-26.
26. Mitchell ES, Woods NF. Pain symptoms during the menopausal transition and early postmenopause. Climateric 2010; 13: 467-478.
27. Whiteman MK, Staropoli CA, Benedict JC, Borgeest C, Flaws JA. Risk factors for hot flashes in midlife women. Journal of Womens Health (Larchmt) 2003; 12: 459-472.
28. Whiteman MK, Staropoli CA, Langenberg PW, McCarter RJ, Kjerulff KH, Flaws JA. Smoking, body mass, and hot flashes in midlife women. Obstetrics and Gynecology 2003; 101: 264-272.
29. Ziv-Gal A, Flaws JA. Factors that may influence the experience of hot flushes by healthy middle-aged women. Journal of Womens Health (Larchmt) 2010; 19: 1905-1914.
30. Thurston RC, Sowers MR, Sutton-Tyrrell K, Everson-Rose SA, Lewis TT, Edmundowicz D et al. Abdominal adiposity and hot flashes among midlife women. Menopause 2008; 15: 429-434.
31. Luoto R. Hot flushes and quality of life during menopause. BMC Womens Health 2009; 18: 13.
32. Williams RE, Levine KB, Kalilani L, Lewis J, Clark RV. Menopause-specific questionnaire assessment in US population-based study shows negative impact on health-related quality of life. Maturitas 2009; 62: 153-159.
33. Skrzypulec V, Dabrowska J, Drosdzol A. The influence of physical activity level on climacteric symptoms in menopausal women. Climateric 2010; 13: 355-361.
34. Daley A, Stokes-Lampard H, Wilson S, Rees M, Roalfe A, Macarthur C. What women want? Exercise preferentes of menopausal women. Maturitas 2011; 68: 174-178.
35. Smith-Dijulio K, Percival DB, Woods NF, Tao EY, Mitchell ES. Hot flash severity in hormone therapy users/non users across the menopausal transition. Maturitas 2007: 58: 191-200.
36. Nelson HD. Commonly used types of postmenopausal estrogen for treatment of hot flashes. JAMA 2004; 291: 1610-1670.
37. Heidi D, Kimberly K, Haney E et al. Nonhormonal therapies for menopausal hot flashes: systematic review and meta-analysis. JAMA 2006; 295: 2057-2071.

