A new and novel strain of influenza A virus (H1N1) emerged from Mexico in the spring of 2009. In April 2009 the Center for Disease Control (CDC) discussed two children infected with H1N1 in Southern California, USA1. By 1 May 2009, the CDC reported that 57 cases of H1N1 had been identified in seven countries outside the USA, including 13 in Canada2. On 11 June 2009 WHO declared this was the start of a H1N1 influenza global pandemic3.
The H1N1 pandemic occurred in two distinct waves, the first occurring in spring 2009, peaking in early June, and the second, larger wave occurring in the fall, peaking in late October and early November4. From its first emergence in April 2009 to 30 January 2010, there were 8791 laboratory confirmed cases of pandemic H1N1 virus in Ontario, with 1843 hospitalizations and 128 deaths5. It is highly likely that this statistic underestimates the true incidence of pandemic H1N1 infection.
The populations most significantly affected by influenza H1N1 differed from the seasonal flu. The highest rate of hospitalizations occurred in patients younger than 14 years and the mean age of death was 51 years. The H1N1 virus seemed to cause significantly less morbidity and mortality in people older than 65 years4,5. Previous studies have shown the average age of death from influenza as 83 years6.
Many studies have been performed to characterize the H1N1 influenza pandemic in different countries. However, no studies could be identified that focused on the incidence and impact in rural emergency departments (EDs). Small hospital EDs provide vital medical services for their communities7,8 and, despite the barriers to accessing care in rural areas, all residents and visitors to rural areas have the right to receive appropriate diagnosis and treatment in a timely fashion9.
Recent studies have shown that rural EDs can provide excellent and timely care for a number of medical conditions10-13. The purpose of this study was to investigate the incident, characteristics and impact of H1N1 on two rural EDs in Southwestern Ontario.
Data were gathered from two rural EDs located in Southwestern Ontario. South Huron Hospital Association (SHHA) is located in Exeter, Ontario. It provides 24 hour ED care, has 19 inpatient beds for a catchment population of over 19 000 people, and receives approximately 10 000 ED visits per year. Alexandra Marine and General Hospital (AMGH) is located in Goderich, Ontario. It also provides 24 hour ED care, has 54 inpatient beds for a catchment of 25 000 people and approximately 16 000 ED visits per year.
An electronic retrospective chart review was performed on all visits to the hospitals' EDs with ICD-10 codes relating to influenza-like illnesses (J11.0, J11.1, J11.8, J18.9, J20.9, J20.88, J09, B34.5 and R50.9). Chart review periods were 1 September 2009 to 1 January 2010 for the H1N1 study group, and 1 September 2008 to 1 January 2009 for the control group. Patients younger than 1 year of age were excluded because the etiology of respiratory disease differs for children in this age group. A standardized chart abstraction form was used to extract information from ED charts. Data abstracted included age, wait time, Canadian Triage Acuity Score (CTAS) category, length of stay (LOS), and disposition. For those patients admitted to hospital we also investigated pre-existing risk factors for H1N1. This chart review was performed following many of the chart review methods previously outlined14,15. The abstracter was trained in proper chart abstraction techniques and used a standardized abstraction sheet. Chart abstraction quality was measured by using a second, blinded abstracter to review 15% of the charts, and ensure inter-rater reliability. Inter-rater agreement was 99.4%. Data were complied and analyzed using Microsoft Excel v 12.0 and OpenEpi software (www.openepi.com). Two-tailed Fisher's exact tests were used to determine statistical significance for the categorical data and unpaired equal variance t-tests were used to determine statistical significance for quantitative data.
Ethics approval for this project was granted by the Health Science Research Ethic Board (HSREB) of the University of Western Ontario (#17213E).
In total, 678 ED charts were reviewed from SHHA and AMGH. There were 546 cases of influenza-like illness (ILI) recorded during the H1N1 pandemic period from 1 September 2009 to 1 January 2010, a 4.1 fold increase from the previous year when 132 cases were recorded from 1 September 2008 to 1 January 2009. Patients with ILI constituted 6.5% (546/8339) of total visits to the rural EDs during the H1N1 pandemic period, compared with 1.6% (132/8125) of the visits during the control period.
During the H1N1 pandemic period, the average number of ILI cases presenting to the rural EDs was 30 cases/week, with a peak incidence between 2 November and 15 November 2009 of 89 cases/week. In the control group, between 1 September 2008 and 1 January 2009 the average number of ILI cases was 7/week, with a peak incidence occurring between 17 November 2008 and 23 November 2008 of 18 cases/week. The weekly incidence of ILI presenting to rural EDs is shown (Fig1).
Half of the cases of ILI that presented during the H1N1 pandemic occurred in patients aged 1 to 20 years. This was significantly higher than the control period. Incidence in the age group 20 to 80 years was approximately 15% per 20 year cohort with 7% of ILI cases seen in patients older than 80 years. The remaining age categories were similar, with the exception of significantly fewer cases seen in the 61 to 80 year group during the pandemic period (Fig2).
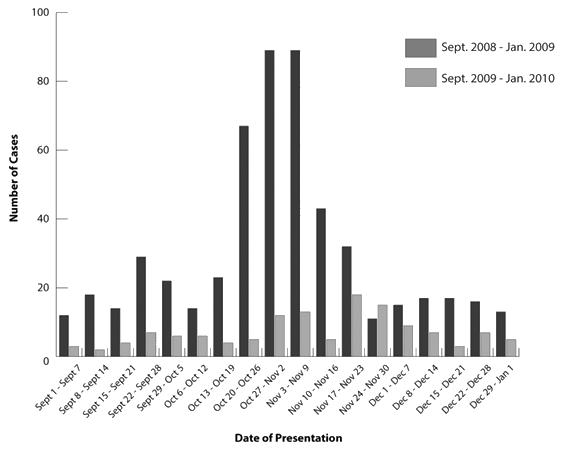
Figure 1: Incidence of influenza-like illness presenting to rural emergency departments, divided by week.
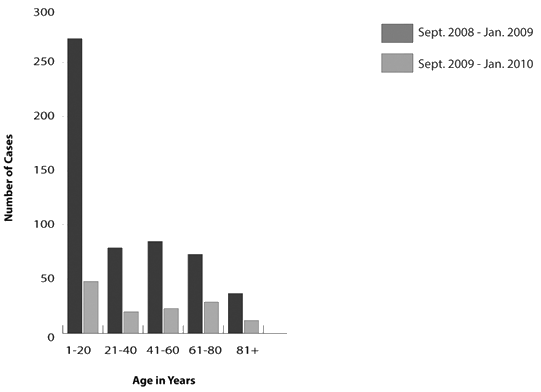
Figure 2: Incidence of influenza-like illness from September 2009 to January 2010,
compared with September 2008 to January 2009, according to age.
The majority of cases of ILI during the H1N1 pandemic were given a CTAS of 4 (n=270, 49%), similar to the control group (n=71, 54%). However, there were significantly more patients categorized CTAS 3 during the pandemic versus the control period (39.0% vs 27.7% p=0.018, CI=95%). When comparing severe ILI cases (CTAS 1, 2 and 3 patients, H1N1 n=225, control n= 43) to less severe ILI cases (CTAS 4 and 5 patients, H1N1 n=316, control n=87), there was no statistically significant difference (p=0.07, CI=95%; Table 1).
Time-to-physician assessment did increase significantly during the pandemic from 41 min to 52 min (p=0.01). However, the mean LOS in the ED did not increase (120 min vs 122 min; Table 1).
For those patients requiring admission the number of risk factors did not show statistical significant differences (Table 1).
There were significant differences in the final diagnostic codes used during the pandemic period versus the control period. The most common code used during the pandemic was J11.1, representing influenza due to unidentified influenza virus with other respiratory manifestations. This was much higher than the control period (32.0% vs 1.4% p<0.01, CI=95%). Diagnostic codes more prevalent during the control period were viral infection, unspecified (B34.9), pneumonia, unspecified organism (J18.9) and fever, unspecified (R50.9; Table 1).
There were no differences in discharge rates, admission rates, transfers to other facilities, unscheduled return ED visits or mortality during the two periods.
Table 1: Cases of influenza-like illness presenting to rural emergency departments,
from September 2009 to January 2010, compared with September 2008 to January 2009
Discussion
There was a 4.1 fold increase in ILI during the H1N1 pandemic period of 2009 in the two rural EDs compared with the control period. This was consistent with data from the Ontario Ministry of Health and Long Term Care16.
The increased burden on rural EDs can be attributed to the increased incidence of ILI and not to an increase in the severity of illness. Measures of severity included the CTAS with CTAS-1 being the most acute and CTAS-5 being least acute. Other measures of severity which did not change were discharge rates, admission rates, transfers to other facilities, unscheduled return ED visits within 72 hours and mortality.
Incidence was significantly increased in the younger age groups. This reflects data from Ontario and around the world17,18. This could be due to a variety of factors including an increase in ILI prevalence in a younger population, pre-existing immunity in the older population, or 'flu fear' driven by the significant media attention during the pandemic18,19.
Interestingly, during the H1N1 pandemic, the proportion of admissions was higher among healthy patients with no risk factors than during the control period. It is possible that H1N1 led to more severe illness in otherwise healthy patients with no underlying risk factors, or that there are risk factors correlated to severe disease that were not identified in our study, such as ethnicity or delayed use of antivirals20. Although ethnicity had been previously described as a risk factor for pandemic H1N1, ethnicity data was not available for this chart review. Alternatively, it is possible that these higher admission rates in patients with no co-morbidities are simply due to the increased incidence of disease in a younger population (age <20 years), who tend to have fewer chronic illnesses. It is important to note that our study uses the incidence of ILI as a surrogate marker of the incidence pandemic H1N1, and the heterogeneity of the study group may influence the results.
The burden of H1N1 translated into increased wait times during the pandemic from 41 to 52 min. There were not specific influenza clinics in these rural communities to divert the less urgent patents from the ED. However, an 11 min increase in time-to-physician assessment may not be clinically significant. This is because the majority of ILI cases were classified CTAS-4, a relatively low severity score, which has a time-to-physician goal of 60 min21. In addition, previous research has shown that rural hospitals meet CTAS guidelines for time-to-physician assessment for all CTAS categories12.
Although the time-to-physician assessment was increased, the mean LOS in the ED was not different. The unchanged LOS suggests healthcare professionals were able to maintain a continuous flow of patients through the ED. The 2 hour LOS for both groups was well within established Ministry of Health performance targets22.
'Returned ED visits' were defined as return visits to the ED for the same condition within 72 hours. The rate of approximately 8% returned ED visits in the control period did not increase during the H1N1 period. Return ED visits for cases of ILI was almost triple the published rate for rural facilities23. It has been shown that improper follow up and insufficient patient education contribute to patients returning to the ED shortly after discharge24-26. The higher return-visit rate in this study suggests more emphasis is needed on proper follow up and improved education.
Admission rates in the H1N1 period compared to the control period did not differ. However, the rate was triple the admission rate for Ontario during the pandemic. This may be because rural hospitals are primary care hospitals and have a lower threshold to admissions. Another factor may be the lower occupancy rates in rural centers compared with regional and urban centers to allow for admissions27.
Although the mortality rate was not different between time periods it was triple the rate for Ontario (1% vs 0.3%). It is not clear why the mortality rate was higher nor whether this is significant. It could represent statistical anomaly due to the small numbers of deaths, or that tertiary centers have a lower mortality rate due to their additional resources.
Limitations
The limitations of this project include incomplete information in charts and coding errors. Cases of H1N1 were not all verified by nasopharyngeal swabs and the ICD-10 codes chosen were not specific for ILI. Access to charts outside SHH and AGMH was not obtained. Access to more rural hospitals across the province or country would have strengthened the conclusions. The incidence of ILI during the pandemic period was compared to the previous year only. The effect of seasonal influenza has a highly variable effect on healthcare systems, and this variability effects the accuracy of our results.
The H1N1 pandemic of 2009 did cause a 5% absolute increase of ILI presenting to the two rural EDs which was significant compared with the previous year. There was also a significant increase in time-to-physician assessment, compared with the previous year. However, the increase did not change the rate of admission and discharge, LOS, transfers to other facilities, unscheduled return ED visits or mortality.
References
1. CDC. Swine Influenza A (H1N1) Infection in Two Children - Southern California, March-April 2009. Morbidity and Mortality Weekly Report 2009, April 24; 58(15): 400-402.
2. CDC. MMWR . Update: Swine-Origin Influenza A (H1N1) Virus- United States and Other Countries 2009, May 1; 58(16): 431-433.
3. WHO. World now at the start of 2009 influenza pandemic, June 11, 2009. Press Statement. Geneva: WHO, 2009.
4. Helferty M, Vachon J, Tarasuk J, Rodin R, Spika J, Pelletier L. Incidence of hospital admissions and severe outcomes during the first and second waves of pandemic (H1N1) 2009. Canadian Medical Association Journal 2010; 182: 1981-1987.
5. Ontario Ministry of Health and Long-Term Care. Ontario Influenza Bulletin 2009-2010 Season, Surveillance Week 4 (January 24, 2010 - Jan 30, 2010). (Online) 2010. Available: http://www.health.gov.on.ca/english/providers/program/pubhealth/flu/flu_09/bulletins/flu_bul_01_20100205.pdf (Accessed 4 June 2012).
6. Bishop JF, Muranae MP, Owen R. Australia's Winter with the 2009 Pandemic Influenza A (H1N1) Virus. New England Journal of Medicine 2009: 361: 2591-2594.
7. Rourke J. Small Hospital Medical Services in Ontario Part 1: Overview. Canadian Family Physician 1991; 37: 1589-1594.
8. Rourke J. Trends in small hospital medical services in Ontario. Canadian Family Physician 1998; 44: 2107-2112.
9. Canadian Association of Emergency Physicians. Recommendations for the management of rural, remote and isolated emergency health care facilities in Canada. Ottawa, ON: Canadian Association of Emergency Medicine, 1997.
10. Vlahaki D, Milne WK. Oligoanalgesia in a rural emergency department. Canadian Journal of Rural Medicine 2008; 13: 62-67.
11. Vlahaki D, Fiaani M and Milne WK. A door-to-needle time of 30 minutes or less for myocardial infarction thrombolysis is possible in rural emergency departments. Canadian Journal of Emergency Medicine 2008; 10: 429-433.
12. Vlahaki D, Milne WK. Meeting Canadian emergency department triage and acuity scale benchmarks in a rural emergency department. Canadian Journal of Rural Medicine 2009; 14: 101-104.
13. Anstett D, Smallfield A, Vlahaki D, Milne WK et al. Door-to-antibiotic time for pneumonia in a rural emergency department. Canadian Journal of Emergency Medicine 2010; 12: 207-211.
14. Gilbert EH, Lowenstein SR, Koziol-McLain J, Barta DC, Steiner J. Chart reviews in emergency medicine research: Where are the methods? Annals of Emergency Medicine 1996; 27(3): 305-308.
15. Worster A, Bledsoe RD, Cleve P, Fernandes CM, Upadhye S, Eva K. Reassessing the Methods of Medical Record Review Studies in Emergency Medicine Research. Annals of Emergency Medicine 2005; 45: 448-451.
16. Public Health Agency of Canada. FluWatch, January 17 to January 23, 2010 (week 3). (Online) 2010. Available: http;//www.phac-aspc.gc.ca/fluwatch/09-10/w03_10/index-eng.php (Accessed 4 June 2012).
17. Chowell G, Bertozzi SM, Colchero MA, Lopez-Gatell H, Alpuche-Aranda C, Hernandez M et al. Severe respiratory disease concurrent with the circulation of H1N1 influenza New England Journal of Medicine 2009; 361: 674-679.
18. McDonnell WM, Nelson DS, Schunk JE. Should we fear "flu fear" itself? Effects of H1N1 infuenza fear on ED use. American Journal of Emergency Medicine 2012; 30(2): 275-282.
19. Hancock K, Veguilla V, Lu X, Zhong Y, Butler EN, Sun H et al. Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. New England Journal of Medicine 2009; 361: 1945-1952.
20. Zarychanski R, Stuart TL, Doucette SS, Elliott L, Kettneret J, Plummer F. Incidence of hospital admissions and severe outcomes during the first and second waves of pandemic (H1N1) 2009. Canadian Medical Association Journal 2010; 182: 1981-1987.
21. Canadian Association of Emergency Physicians. Canadian Emergency Department Triage and Acuity Scale Implementation Guidelines. Triage and Acuity Scale - Category Definitions. (Online) 1999. Available: http://www.cjem-online.ca/v1/n3/PaedCTAS/p4#LessUrgent (Accessed 4 June 2012).
22. Ministry of Health. Improving Access to Emergency Care: Addressing System Issues. (Online) 2006. Available: http://www.health.gov.on.ca/english/public/pub/ministry_reports/improving_access/improving_access.pdf (Accessed 4 June 2012).
23. Foran A, Wuerth-Sarvis R, Milne WK. Bounce Backs in a Rural Emergency Department. Canadian Journal of Rural Medicine 2010;15(3): 108-112.
24. Keith KD, Bocka JJ, Kobernick MS, Krome RL, Ross MA. Emergency department revisits. Annals of Emergency Medicine 1989; 18: 964-968.
25. Kelly AM, Chirnside AM, Curry CH. An analysis of unscheduled return visits to an urban emergency department. New Zealand Medical Journal 1993; 106: 334-336.
26. Lerman B, Kobernick MS. Return visits to the emergency department. Journal of Emergency Medicine 1987; 5: 359-362.
27. Ministry of Health and Long-Term Care. Healthcare Indicator Tool - Facility Type Comparison. (Online) c2010. Available: https://hsimi.on.ca/hdbportal/ (Accessed 1 September 2011).
