Live kidney donors have become a critical source of organs for renal transplantation due to the scarcity of organs from deceased donors1. Indeed, in Australia and internationally, live kidney donation is assuming an increasingly prominent role in kidney transplantation programs2-4. In Australia, live kidney donation is a routine and common procedure with low morbidity and excellent long-term results, and with better clinical outcomes than using kidneys from deceased donors5.
There is limited research conducted on the experience of live renal transplantation from the perspectives of the donors6,7. Of specific relevance to this paper, there has been no research exploring the financial impact on the live renal donor in terms of testing, hospitalisation and surgery for kidney removal (known as nephrectomy). The only mention of financial issues in relation to live renal transplantation is the recipients' concerns in relation to monetary payment for the gift of a kidney and the recipients' desire to pay for the costs associated with the nephrectomy7,8. The discussion in this article posits a new direction in live renal donor research; that of understanding the financial impact of live renal donation on the donor to inform health policy and supportive care service delivery. As the findings indicate that the major financial difficulties were experienced by regional, rural and remote donors, the research makes a contribution towards deepening the understanding of the challenges faced by non-metropolitan people who have to relocate for specialist medical care. The findings reported in this article are from the first interview (T1) of a set of interviews (T1-T4) conducted over time with each participant from a longitudinal, qualitative study that explored the experience of live renal donors who were undergoing a nephrectomy at the Renal Transplantation Unit at the Princess Alexandra Hospital (PAH), Brisbane, Queensland.
Renal transplantation at the Princess Alexandra Hospital
The Princess Alexandra Hospital (PAH) is a 600 bed, public hospital located in Brisbane, Australia. The PAH Renal Transplant Unit adopts a multi-disciplinary and collaborative approach to patient care, providing comprehensive pre- and post-transplantation services. The PAH Renal Transplant Unit performs over 110 renal transplants each year, of which 40 are from living kidney donors. The Renal Transplant Unit maintains the highest survival rate for kidney transplants within Australia and New Zealand. With close affiliation with the hospital's Urology Department and Nephrology Department, the Renal Transplant Unit provides intensive short- and long-term follow-up for renal transplant recipients and their families9. In Queensland, there are 1592 patients on dialysis out of a total of 8528 in Australia; at the PAH alone, there are 397 patients on dialysis10. By April 2009, the number of transplants performed at the PAH had reached 3000, with the hospital's first living kidney donor transplant having occurred in 198110.
The participants were enrolled through the project officer for the study, who was under contract with CQUniversity and, thus, independent of the PAH. The project officer was provided with a list of potential donors who were being tested for compatibility with the recipient, along with their telephone numbers. This information was provided by the transplant coordinator at the hospital, who had gained verbal consent for this from each potential participant. All potential participants had been informed about the study from the hospital, both verbally from the coordinator and in writing through a letter outlining the details of the study. The participants were enrolled from this list through an initial telephone call, followed by the project officer providing a written project description of the project and an invitation for voluntary participation in the research. At this stage, signed consent forms from the participants were collected and enrolment occurred. Participants were not screened for the study per se, but all participants had to undergo both clinical and psycho-social assessment to be accepted as suitable to be a living kidney donor in the PAH Renal Transplant program. The psycho-social assessment, conducted by the liaison psychiatrist and social worker attached to the renal unit, explores through interviews the individual's decision making with regards to that individual's desire to donate. Prior to interviewing for the research, participants were again informed of their ethical rights (eg informed consent, confidentiality, right to withdraw).
A total of 20 participants (n=20) were enrolled at T1 (pre-transplant interview) and followed-up longitudinally for the study over three points in time: time 2 (T2) - 3 months post-transplant; time 3 (T3) - 1 year following T2; time 4 (T4) - 1 year following T3. One of the participants died during the study, leaving the final group of 19 at T4. All participants were enthusiastic about their involvement in the study, chose to participate through telephone interviews, noted the time for the next interview at the close of each interview, and were welcoming when contacted by telephone for subsequent interviews. The T1 enrolment procedure was complicated by the fact that it was difficult to identify the ultimate donor when there were several potential donors for the one recipient under assessment or where the potential participant under assessment was eventually found to be clinically incompatible. The waiting time for the transplant was in many cases immediate once the donor was assessed as compatible. Thus, in order to obtain the pre-transplant interview for the correct donor who would undergo the transplant and be followed up, it was necessary to interview some participants who did not progress to the transplant stage. Thirteen (n=13) interviews were conducted with potential T1 participants who did not become donors.
The participants were all of adult age and had the following range of relationships with the transplant recipient: daughter (n=4), son (n=1), husband (n=6), wife (n=4), sister (n=2), nephew (n=1), partner (n=1) and friend (n=1). Because the participants were enrolled from a small, identifiable group at the hospital, the informed consent procedures gave a strict commitment to confidentiality and a guarantee that no further identifying information would be presented or published with the findings. Hence, further demographic descriptions will not be provided in order to protect the identity of the participants.
Research design
An open-ended, exploratory qualitative design was utilised for the study. Qualitative research is used to provide in-depth insights on consumer experiences and perceptions and, thus, is an effective means of gaining rich insights into the experience of living kidney donors11,12.
Interviews
The exploration of the renal donors' experience was conducted through an iterative, qualitative research methodology using open-ended interviews conducted at the time and location chosen by each participant. The telephone interviews were conducted by an experienced researcher with a background in psycho-social health research employed by CQUniversity and, thus, independent of the hospital. All participants chose to do the interviews through speakerphone.
One of the topics explored during the TI interviews was the financial impact of the testing and nephrectomy. The interviews lasted for approximately 1 hour and were audio-recorded. The interviews were transcribed verbatim by a research assistant independent of the hospital.
Analysis
The language texts were entered into the QSR NUD*IST (N5 1995; www.qsrinternational.com) computer program and analysed thematically. All of the participants' comments were coded into 'free nodes', which are category files that have not been pre-organised but are freely created from the data. The list of codes was then transported to a Microsoft Word document (Word 97) and organised under thematic headings. The coding was established by a qualitative researcher and completed by the project officer, who had extensive experience with coding qualitative data. There was complete agreement between researcher and project officer on the coding and emergent themes. The findings from the codes developed from the TI pre-transplant interviews in relation to the financial impact on the donor are presented in this article. Further findings on different topics from the longitudinal study based on the same methodology are currently being published13.
Ethics approval
The University Ethics Committee and the Queensland Health Department, Human Research Ethics Committee approved the study (no. H07/07/068).
There were two main groups of participants in terms of financial impact: those who experienced financial strain from the costs associated with nephrectomy and those who were financially well off and, thus, did not experience financial concerns (Table 1).
Table 1: Financial impact on live donor

The major factors associated with financial concern were the cost of testing, the extra costs for donors living in non-metropolitan locations, and the loss of income during nephrectomy and recovery (Table 2). For donors living in the regional, rural and remote areas there were extra costs associated with the fact that bulk billing (no cost to the patient for practitioner's service) is not always available, that individuals have to pay up-front first, that free testing at local public hospitals is not available in some areas and that non-metropolitan donors have to fund the extra cost of travel and accommodation when relocating for the nephrectomy to the specialist metropolitan hospital. There were also financial difficulties associated with employment. These included taking time out for those who were self-employed; loss of income during operation and recovery; lack of income because of lack of sick leave or long periods off work from a loss of job post-transplant; and the double financial impact from loss of income when recipient and donor were partners dependent on each other for income.
There were a number of buffers that deflected negative financial impact for donors in regards to the cost of testing and nephrectomy, and loss of income (Table 3). A major buffer was the fact that both testing and the nephrectomy were funded by the public hospital system. The reasons why individuals chose to avoid this financial benefit to go into the private system are outlined (Table 4). It is important to note that one of the reasons for choosing to do testing in the private system for non-metropolitan donors was that public hospital testing was not locally available.
Table 2: Financial concerns associated with nephrectomy
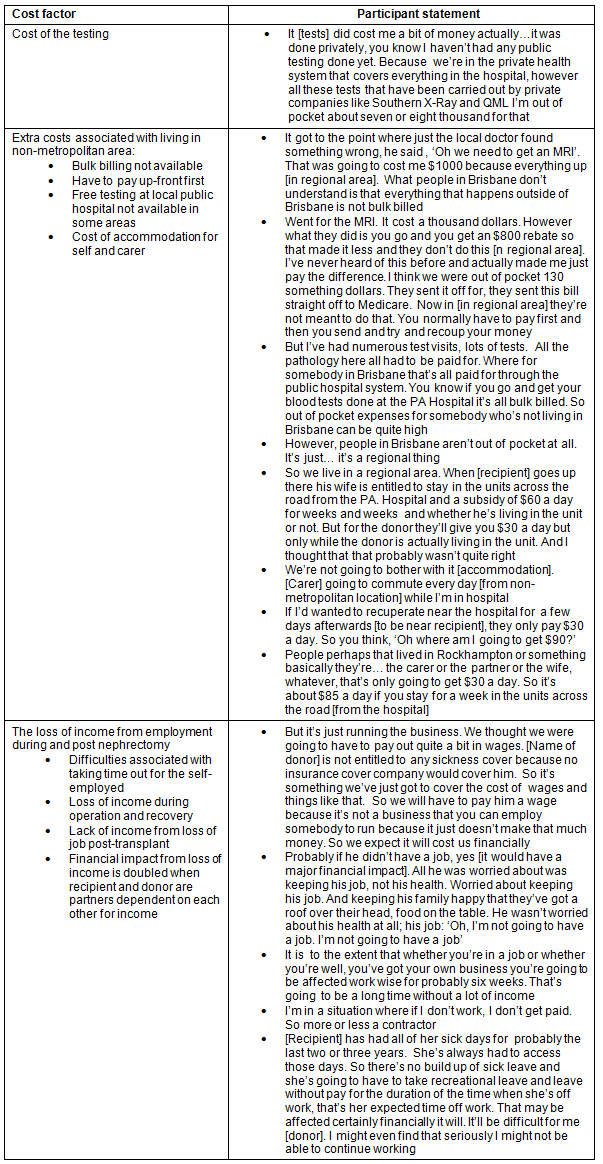
Table 3: Buffers reducing the impact of financial stress
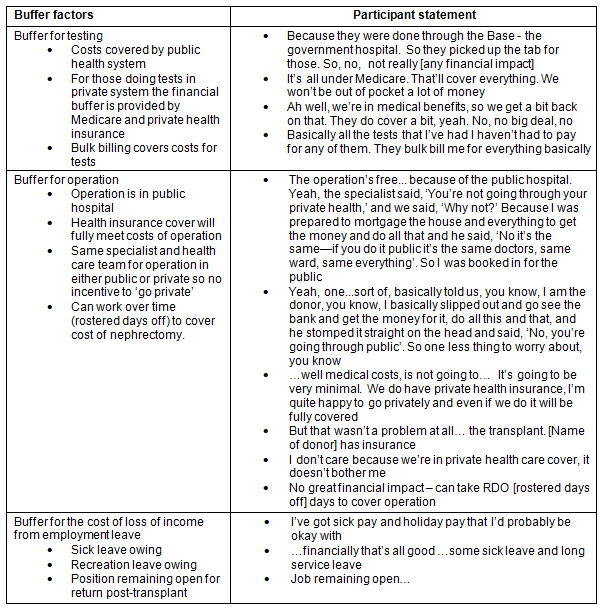
Table 4: Reasons for choosing private health system for testing
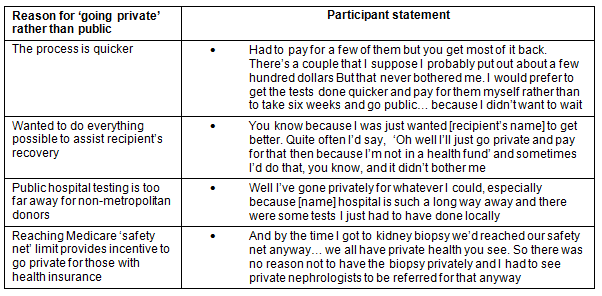
Individuals did express gratitude for the fact that live renal transplant is to a degree publicly funded, for example:
I'm always conscious of the fact that the public purse is paying for this very expensive operation. I'm very grateful that it is for us not costing us anything. If I'm out of pocket you know a couple of thousand dollars for flights and tests and things like that, in the big scheme of things we're still getting off. In the big picture we're still getting off very lightly.
However, even for those individuals who were well off and without financial strain, they acknowledged that the costs associated with being a living renal donor would potentially be too expensive for those on limited finances. For example:
But a normal ... like my son could never have done it because they live week to week. I mean they've got a mortgage, a baby...
But the average person could not possibly do it.
You may find that some people that are prepared to do it may find that they can't afford to do it (because of loss of income during operation and recovery).
The financial difficulties were considered particularly acute for those living outside the metropolitan area, for example:
...a lot of people out there that are prepared to donate an organ, if they aren't all that wealthy and they live in an out of Brisbane area, they are going to find that if they've got to pay all the accommodation costs themselves that could impact on them quite a bit.
A recommendation documented in the research is that addressing the financial impact for donors may help to increase the number of people who are prepared to become a living renal donor, particularly for those outside the metropolitan area, for example:
Oh well I think that probably from the perspective of increasing the number of donors or whatever ... For live donors that['s] probably a little bit more support for them financially, might at least bring them up to square one.
If there was some sort of financial help it would (help) ... because the costs are even though it's through the public system there are costs and particularly for a person that has to drive up from [name of non-metropolitan area] and all of that and like having the time off work. Even though I have got some sick leave the rest of it is going to be without pay. It's not even like an illness you have, it's something you are contributing to somebody else so your costs should be totally covered.
Discussion
At present there is limited work documenting the financial factors associated with live renal transplantation. A study by Coorey and associates14 demonstrated that a lack of financial resources can be a substantial barrier to pre-emptive kidney transplantation. However, on the financial impact of live renal transplant, the work of Coorey and associates is not specifically focused on regional issues and is from the recipients' perspective14. The work of White and associates15 focused on the financial and resource issues associated with global equity in the provision of renal replacement therapy. Bond16 points out that cost-containment will be an ongoing issue in the environment of fiscal constraint in which renal transplant centres operate. Although all of these issues are important, they do not address the financial concerns of live renal donors. With regards to the topic of the present article, there is no research available on the financial impact of live renal donation for individuals living in non-metropolitan areas. Thus, this article is a seminal work in the area that raises a highly important and relevant concern for the long-term future of live renal donation. The hope and expectation is that this preliminary work will encourage more detailed research in different geographical locations and populations.
Known as the 'rural disadvantage', there is now acknowledgement in the literature of the socioeconomic difficulties, inequitable access to services and geographic factors that make access to specialist medical care problematic for those in non-metropolitan areas17. The findings reported in this paper affirm this disadvantage by demonstrating that it is the live renal donors in non-metropolitan areas who are reporting financial concerns in relation to testing, hospitalisation and surgery for nephrectomy.
Australian-based research indicates that there can be considerable financial distress for non-metropolitan families that have to relocate for specialist medical care18,19. Although most of the research completed in this area is in relation to cancer patients, it is now well documented that rural and remote residents often have to take time off work, travel long distances, and pay for accommodation and other relocation expenses in order to access specialist care in a metropolitan area19-22. The financial hardships for non-metropolitan live renal donors documented in the present findings include that bulk billing is not always available, individuals have to pay up front first, free testing at local public hospitals is not available in some areas, non-metropolitan donors have to fund the extra cost of travel and accommodation when relocating for the nephrectomy to the specialist metropolitan hospital, and return trips to the metropolitan hospital have to be funded for follow-up care.
Hiller and associates23 noted that live renal donors in the USA expressed concern about the number of days off work that they would require. The present findings posit a range of work-related financial concerns for live renal donors, including difficulties associated with taking time out for the self-employed, loss of income during operation and recovery, lack of income from a loss of job post-transplant, and the double financial impact from loss of income when recipient and donor are partners dependent on each other for income.
It is a concern that the focus of research to date has not included the financial impact of nephrectomy in the exploration of the live renal donor's experience. Recent UK research by Mazaris and associates24 indicates that any financial reward, even as compensation of expenses, is a controversial ethical topic from the health professional's perspective. It is acknowledged that one of the difficulties in advocating for financial support for live renal donors is the conflation of the issue with the discussion of payment or reward for the donation of a live kidney7,8. However, as noted by Walsh, the investigation of the live renal donor's experience is important so that potential donors are supported appropriately by the healthcare system25. It is argued in this article that for all live renal donors, and especially for those living in non-metropolitan areas, it is essential to understand the financial implications of nephrectomy. Knowledge of the financial impact is essential to ensure that costs are not a barrier to the decision to become a live renal donor, there is equity in the opportunity to participate as a donor, appropriate practical support is provided to donors, and issues of relocation for non-metropolitan donors are addressed.
Live renal transplantation is an important new direction in medical care that has excellent long-term results for individuals diagnosed with end-stage renal disease. An essential element of the transplantation procedure is the voluntary donation of a healthy kidney by the live renal donor. Such an altruistic gift, which has no personal health benefit for the donor, is to be applauded and supported. The present research demonstrates that for some donors, particularly those living outside the metropolitan area, the gift may also include a range of financial costs to the donor. It is the hope and expectation that the reporting on these costs will encourage further qualitative and quantitative work in this area and that the findings will be used for future health policy and service delivery considerations.
Acknowledgements
The authors thank Dr Paul Pun, Ms Melinda Jesudason, Ms Mary Anne Patton, Ms Bo McGrath, Ms Gayle Frohloff, Ms Alana Cresswell, Ms Rachel Polley, Mrs Elaine Phillips and CQUniversity for funding through a University Merit Grant.
References
1. Steinberg D. An 'opting in' paradigm for kidney transplantation. American Journal of Bioethics 2004; 4(4): 4-14.
2. Brook N, Nicholson M. Minimally invasive surgery for live kidney donors: techniques and challenges. Progress in Transplantation 2005; 15(3): 257-263.
3. Crombie A, Franklin P. Family issues implicit in living donation. Mortality 2006; 11(2): 196-210.
4. Levidiotis V. Live kidney donors - assessment and follow up. Australian Family Physician 2009; 38(5): 316-320.
5. Woodroffe C, Ferrari P. The Western Australian experience with a kidney paired donation programme. Transplant Nurses' Journal 2009; 18(2): 5-8.
6. Nolan M, Walton-Moss B, Taylor L, Dane K. Living kidney donor decision making: state of the science and directions for future research. Progress in Transplantation 2004; 14(3): 201-209.
7. Yi M. Decision-making process for living kidney donors. Journal of Nursing Scholarship 2003; 35(1): 61-66.
8. Pradel F, Mullins C, Bartlett S. Exploring donors' and recipients' attitudes about living donor kidney transplantation. Progress in Transplantation 2003; 13(3): 203-210.
9. Queensland Government, Queensland Health, Princess Alexandra Hospital Renal Transplant Unit (PAHRTU). (Online) 2007. Available: http://www.health.qld.gov.au/pahospital/research/rtu.asp (Accessed 30 October 2012).
10. Queensland Renal Transplant Service. Kidney transplantation - what you need to know. Brisbane, QLD: Queensland Health, 2010.
11. Holloway I. A-Z of qualitative research in healthcare, 2nd edn. Oxford: Blackwell Publishing, 2008.
12. Patton M. Qualitative research and evaluation methods, 3rd edn. Thousand Oaks, CA: Sage, 2002.
13. McGrath P, Pun P, Holewa H. Decision-making for live donor renal transplantation: findings from Australia. Mortality 2012; 17(3): 201-220.
14. Coorey G, Paykin C, Singleton-Driscoll L, Gaston R. Barriers to preemptive kidney transplantation: a survey of people with chronic kidney disease finds that many see transplantation as an option of last resort. American Journal of Nursing 2009; 109(11): 28-38.
15. White S, Chadban S, Jan S, Chapman J, Cass A. How can we achieve global equity in provision of renal replacement therapy? Bulletin of the World Health Organisation 2008; 86(3): 229-237.
16. Bond M. Health promotion and disease prevention in kidney transplant recipients. Journal of Transplant Coordination 1998; 8(4): 221-224.
17. Francis K. Health and health practice in rural Australia: Where are we, where to from here? Online Journal of Rural Nursing and Health Care 2005; 5: 1.
18. McGrath P. It's horrendous - but really, what can you do? Preliminary findings on the financial impact of relocation for specialist treatment. Australian Health Review 2001; 23(3): 94-103.
19. McGrath P, Holewa H, Munro A. Government funded travel and accommodation assistance: learning from inter-country research. Journal of Rural and Tropical Public Health 2011; 10: 87-94.
20. McGrath P. Accommodation for patients and carers during relocation for treatment for leukaemia: a descriptive profile. Journal of Supportive Care in Cancer 1999; 7(1): 6-10.
21. McGrath P, Seguerra J. The patient transit assistance scheme: a consumer's perspective. Australian Journal of Rural Health 2000; 8: 232-238.
22. Smith T. A long way from home: access to cancer care for rural Australians. Radiography 2012; 18(1): 38-42.
23. Hiller J, Sroka M, Weber R, Morrison A, Ratner L. Identifying donor concerns to increase live organ donation. Journal of Transplant Coordination 1998; 8(1): 51-54.
24. Mazaris E, Warrens A, Papalois V. Ethical issues in live donor kidney transplant: views of medical and nursing staff. Experimental and Clinical Transplantation 2009; 7(1): 1-7.
25. Walsh A. Living kidney donor experiences: implications for counselling. EDTNA/ERCA Journal of Renal Care 2004; 30(4): 196-200.

