Diarrhoea is a major health problem1. It is usually a symptom of an infection in the intestinal tract, which has a variety of causative agents including viruses, bacteria and parasites2,3. Diarrhoeal infection spreads through the ingestion of contaminated food or drinking-water, or person-to-person as a result of poor hygiene. There are three clinical types of diarrhoea: (i) acute watery diarrhoea which lasts several hours or days and includes cholera; (ii) acute bloody diarrhoea, also called dysentery; and (iii) persistent diarrhoea that lasts 14 days or longer1,4.
The vast majority of deaths from diarrhoea are among children under 5 years of age living in low- and middle-income countries5. Diarrhoeal disease due to unsafe water and lack of sanitation is the greatest cause of morbidity and mortality in under-five children in the world, especially in poor countries5,6. A child dies every 15 seconds from diarrhoea caused largely by poor sanitation and a contaminated water supply7. As a child's immune system is progressively compromised with each bout of diarrhoea, related illnesses kill millions more indirectly. In developing countries, approximately 2 million people, the vast majority of whom are under-five children, die from diarrhoea each year6. Nearly 90% of diarrhoea is attributed to unsafe drinking water, inadequate sanitation and poor hygiene6.
It is widely recognized that exposure to diarrhoea pathogens in developing countries is associated with the age of the child, quality and quantity of water, availability of toilet facilities, housing conditions, level of education, household economic status, place of residence, feeding practices, and the general sanitary conditions (personal or domestic hygiene) in the vicinity of the house8-12. Socioeconomic factors may directly and indirectly affect environmental, behavioral, nutritional, and demographic risk factors, with the exception of age and sex9.
Diarrhoea alone kills more children than AIDS, malaria, and measles combined10. Recent estimates indicate that the two-week period prevalence of diarrhoea in under-five children in Ethiopia is approximately 24%13. Morbidity-Mortality-and Treatment (MMT) surveys conducted in Ethiopia at various times have revealed five diarrhoeal episodes per child per year, and the two-week incidence to be 16%. Studies conducted in central, rural Ethiopia have revealed diarrhoea to be one of the common causes of under-five mortality, accounting for approximately 8.4% to 27% of all deaths4.
Diarrhoea is also responsible for 25% to 75% of all childhood diseases, and accounts for approximately 14% of outpatient visits and 16% hospital admissions in Ethiopia13. In addition to excess mortality and morbidity, diarrhoea predisposes children to malnutrition, which makes them highly susceptible to other infections, and this has been found to be a major contributor to illness and death, particularly among children in Sub-Saharan Africa9,14. Behavioral factors associated with acute childhood diarrhoea include lack of hand-washing, poor infant and young child feeding practices, and lack of child immunizations8,15-19. The objective of the present study was to identify socioeconomic, environmental and behavioral determinants of acute childhood diarrhoea which can be modified to improve child health.
Study area and period
The study was conducted in rural kebeles (or neighborhoods, the smallest administrative unit of Ethiopia) of Derashe District, Segen Area People's Zone, Southern Nations Nationalities and Peoples Region (Fig1), from January to February 2012. Derashe District is located 550 km from Addis Ababa and at the time of the study had an estimated population of 119 569 with 1:1 sex ratio, 27 860 women of reproductive age and 18 653 under-five children. Of the environmental health conditions in the district, there is 85% latrine availability and 41.9% of the population have access to an improved water supply.
Study design
This was a community based, unmatched case-control study supplemented with Focus Group Discussion (FGDs). A 'case' was defined as a child under-five years of age, resident in a Derashe rural area, with a report of diarrhoea by mother and/care-taker in the 2 weeks preceding the survey. Control was a child under-five years of age without diarrhoea in the preceding two weeks, randomly chosen from the resident population in the rural kebele. Focus group discussions were conducted to supplement the results from the quantitative data collection.
Study population and sample detail: The study population was children under-five years of age. Children with persistent diarrhoea were excluded from the study.
The sample size calculation was based on the following assumptions: P1=proportion of diseased with disposal of refuse in pit; P2= proportion of non-diseased with disposal of refuse in pit. From a similar study conducted in Nekemte town with refuse disposal method as the main predictor of outcome (diarrhoea), the sample was 42.86% for cases and 61.47% for control13. Therefore, P1=0.4286, z1=1.96 (95% CI) and P2=0.6147, z2=0.84 (power of 80%) the proportion of case and control was assumed to be 1:2 With a design effect of 2 and 10% non-response rate, the total sample was estimated using Epi Info v3.5.3 software (http://wwwn.cdc.gov/epiinfo/) as 612 (cases=204, controls=408).
Multi-stage sampling procedure was employed, first by selecting five kebeles from the fifteen using a lottery method. Then all households with under-five children in the selected kebeles were registered through a house-to-house survey by 10 trained enumerators, in order to register all under-five children with diarrhoea or without diarrhoea. After identifying cases and controls by the census, the sample was selected by using a simple random sampling technique. Purposive sampling was used to select participants for qualitative study/FGDs in order to have participants who could provide adequate information.
Study variables
The dependant variable of the study was the occurrence of acute diarrhoea. Independent variables included socioeconomic and demographic characteristics such as income of a family, maternal age, age of child, housing condition, family size, and maternal education. Environmental factors were the availability and utilization of a latrine, waste disposal, and types of water source. Behavioral factors included patterns of hand-washing at critical times, water storage, water treatment, breast feeding status, duration of breastfeeding, and time of introducing supplementary feeding. The definition of some variables follows:
- Improved water source: protected spring, protected dug well and piped water
- Unimproved water source: pond, river, unprotected dug well and unprotected spring
- Hand-washing at critical times: hand-washing after visiting toilet, before eating food and after feeding children.
- Adult member defecation: latrine utilization by adult members of the household, both at home and when outside the home.
Data methods and analysis
Data collection: Quantitative data were collected from the study population using a pre-tested, structured questionnaire via face-to-face interviews in the local language. The qualitative data were collected by using an interview guide.
Data quality assurance: A properly designed and pre-tested questionnaire was used. Interviewers and data clerks were trained and closely supervised during data collection and entry. Double data entry was used to ensure data quality.
Data analysis: Quantitative data were entered into a pre-drafted coding sheet in Epi Info by two data clerks. All data analysis was performed using SPSS v16.0 (www.spss.com). Binary logistic analysis with enter method calculating odds ratios (OR) and 95% confidence intervals (CI) was used to estimate the association between the dependent and independent variables. Statistical significance was set at α ≤0.05. In an attempt to identify the relative effects of explanatory variables on the outcome variable, hierarchical multivariable analysis was applied. Explanatory variables with p-value <0.2 were entered into the final regression model based on the likelihood ratio for further analyses in the three different models.
The FGD were tape recorded. Qualitative data were collected using an interview guide that asked participants' perceptions of different determinants of diarrhoeal diseases. The interview guide was used to guide the flow of discussion and collect data. Careful attention was given to establish the frequency of the occurrence of themes, phrases and expressions as participants described their opinions relative to the specific research questions. The tape recorded data were analyzed according to selected themes based on the guide, and summarized manually according to thematic analysis. Open code software was used to facilitate the analysis. The written-notes and tape recorded data were transcribed line by line and translated from the local language (Dirayta) into Amharic and then to English.
Ethics approval
The ethical approval and clearance for this research study was obtained from Haramaya University College of Health Sciences Institutional Research Ethics Review Committee. At all levels, officials were contacted and permission from administrators was secured. Participants were provided with an explanation of the purpose of the study and its procedures, and confidentiality was assured. Both written and verbal consent was obtained from the study participants.
Socio-economic and demographic characteristics
Among households with children under the age of 5 years there was a response rate of 96.7% and 199 cases and 393 controls were included in the study. The remaining 20 (5 cases and 15 control) households were non-respondent. Four hundred and eighty-seven (82.3%) interviews were conducted with mothers of children under 5 years of age and the rest with caretakers.
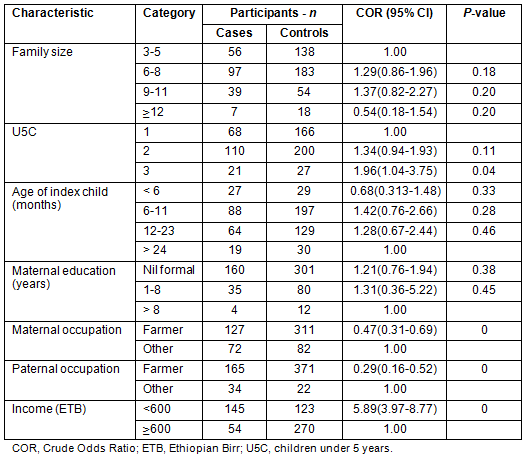
The occurrence of acute childhood diarrhoea had statistically significant associations with the number of under-five children in the household (OR: 1.96, CI: 1.04-3.57), mother's occupation (OR: 0.47, CI: 0.31-0.69), and family income (OR: 5.89, CI (3.97-8.77). Educational status of mothers or care takers, age of index child and ethnicity were not statistically significant (Table 1).
Table 1: Sociodemographic and economic characteristics of study participants, Derashe District, South Ethiopia, 2012
Environmental factors associated with diarrhoea
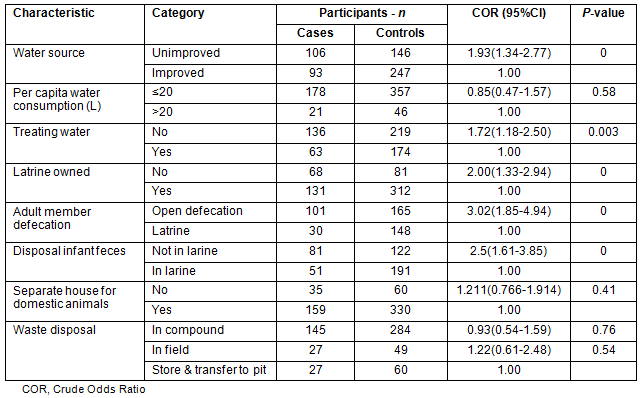
Statistically significant associations were found between acute childhood diarrhoea and sources of water (p<0.001), availability of home-based water treatment (p=0.003), latrine ownership (p<0.001), patterns of adult member defecation (p<0.001) and disposal of infant feces (p<0.001) (Table 2).
Table 2: Environmental factors related to acute diarrhoea among study participants in Derashe District, South Ethiopia, 2012
Behavioral factors associated with diarrhoea
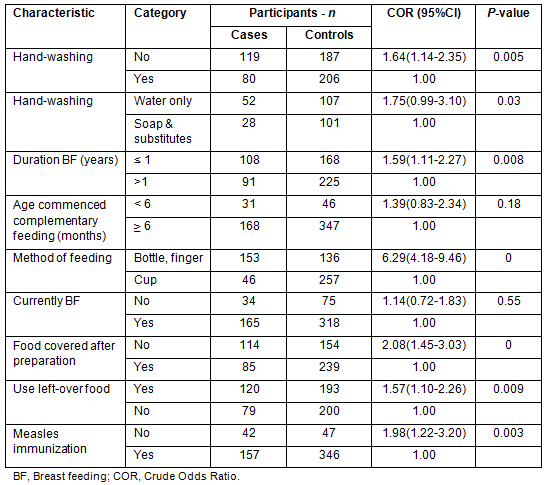
The acute childhood diarrhoea had significant associations with duration of breast feeding of children (p=0.008), hand-washing (p=0.005), covering of food after preparation (p<0.001), consumptions of left-over food stored at room temperature (p=0.009) and measles vaccination (p=0.003) (Table 3).
Table 3: Behavioral factors associated with acute diarrhoea in bivariate analysis, Derashe District, south Ethiopia, 2012
Qualitative results
Three FGDs of 12 participants in each group were conducted with parents who could give adequate information. Qualitative data analysis revealed that perceived causes of diarrhoea were: child eating dirt, tooth eruption, 'falling of the pancreas' and intestinal parasites. Eating dirt was among the most frequently mentioned causes of diarrhoea, explained by participants as occurring when a child of 5-6 months starts to play on the ground and is likely to eat dirt frequently. Participants also said that 'a child develops diarrhoea after five or six month of age when the child is teething. If the tooth is extracted then the diarrhoea will stop'. 'Fallen pancreas' was described as a shift in the position of pancreas that occurs during a fall. When this occurs a child will cry and experiences pain when the legs and abdomen are touched. If the pancreas is returned to its position by traditional healers, both the diarrhoea and the pain cease.
Concerning care of a child with diarrhoea, a number of issues were raised. A female participant said that 'since diarrhoea is caused by worms that cause liquid stool, restricting fluids can prevent it'. Participants also recommended taking the child to a traditional healer to discover the main cause of diarrhoea and to obtain the required care. Taking a child with diarrhoea to a health facility in the first 6 months of life is generally not accepted because the cause of diarrhoea is linked to teething. However, after 6 month of age the child can be taken to a health facility.
Regarding prevention of diarrhoeal diseases and the role of water treatment, one participant said:
It is not easy to control diarrhoea using treating water because some of the germs may not be killed with boiling; use of chemical alters the taste of water and it could not be used in this culture.
Multivariable analysis
Hierarchical logistic regression technique was used to assess the relative effect of the explanatory variable on the outcome variable. To avoid an excessive number of variables and unstable estimates in the subsequent model, only variables with a p-value <0.20 were kept in the analyses.
Three nested logistic models were used to estimate the effects of explanatory variable on the outcome variable:
- Model 1: examined the joint effects of socioeconomic and demographic factors
- Model 2: used sociodemographic factors with p-value <0.20 in Model 1, and environmental factors
- Model 3: built on variables with p-value <0.20 in Model 2 and included behavioral factors.
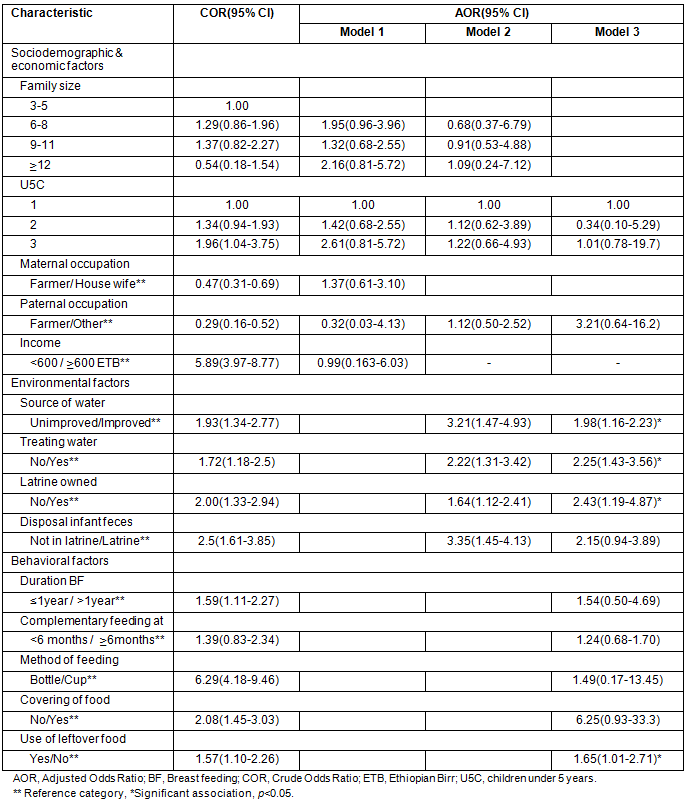
From the total variables entered in the multivariable regression, four were found to have significant independent association with the diarrhoea (Table 4):
- The odds of developing diarrhoea was 1.98 times higher among children whose family used an unimproved water source compared with the children of families who used an improved water source (adjusted [A] OR:1.98, 95% CI [1.16-2.23]).
- The odds of developing diarrhoea was 2.25 times higher among children whose families did not treat drinking water, compared with children whose families treated water for drinking (AOR:2.25, 95% CI [1.43-3.56]).
- The odds of developing diarrhoea was 2.43 times higher among children of families who had no latrine, compared with the children of families who had a latrine (AOR: 2.43, 95% CI [1.19-4.87]).
- Children who consumed left-over food stored at room temperature were 1.65 times more likely to have diarrhoea compared with children who did not consume left-over food stored at room temperature (AOR:1.65, 95% CI [1.01-2.71]).
Table 4: Independent predictors of acute diarrhoea in Derashe District, South Ethiopia, 2012
Discussion
This study found the determinants of acute diarrhoea to be the source of household water, availability of home-based water treatment, latrine ownership and the consumption of left-over food stored at room temperature.
An unimproved water source is among the potential sources of transmission of diarrhoeal diseases. The probability water source contamination depends on whether or not the source is protected. Households using unprotected water sources were three times more likely to have a child with diarrhoea. This finding is in agreement with a study conducted in Gonder19 but it contradicts a study conducted in the Gojam zone12 (both locations are in northern Ethiopia). This may be due to the use of water from both protected and unprotected sources, contamination during transport and storage or a lack of homogeneity in water sources in the Gojam study.
Availability of home-based drinking water treatment was an independent predictor of diarrhoeal morbidity in this study. Children whose families used home-based drinking water treatment such as boiling, the use of chemicals (eg aqua tabs and wuha-agar) and/or filtering were found to have lower odds of getting diarrhoea compared with the children of families not treating water. Because collected water may be contaminated during collection, transportation and storage, this may increase the risk of diarrhoeal diseases. This finding is in agreement with study from Kenya20 but contradicts previous studies in Ethiopia12. This may be due to varying methods of home-based water treatment, and overall differencse in the sanitation of the environment.
In the present study children from households without toilet facilities were more likely to develop diarrhoea compared with children from households with latrine facilities. The presence of toilets increases the chance of safe disposal of feces, one way to decrease contact between the causative organisms of diarrhoea and the host. This finding was similar to other studies8,14,20-23.
The consumption of left-over food stored at room temperature is also an independent predictor of acute childhood diarrhoea due to contamination with and the proliferation of diarrhoea-causing bacteria. The present study cases were almost twice as likely to consume left-over food stored at room temperature than controls.
It has long been established that bottle-fed children are at greater risk of diarrhoea than those who are breast fed, due to milk contamination. Even though it was not statistically significant in the multivariable analysis, the odds of bottle-fed children having diarrhoea was approximately one and a half times greater than for children who were not bottle fed. Bottle-feeding was significantly associated with diarrhoeal morbidity in the bivariate analysis. Similar findings have been observed in India and Nekemte, western Ethiopia8.
The number of under-five children and family size were not independently associated with diarrhoeal morbidity. This is in agreement with a study from Jimma in west Ethiopia21 but contradicts the finding from the locations Meskanena Mareko Woreda (central Ethiopia) and Gojam Hullet Ejju Ense Woreda (north Ethiopia) where greater family size was associated with diarrhoeal morbidity (OR=2.07 & OR=2.3, respectively)12,23. This might be due to differences in maternal attention, general living conditions and differences in study design. It is known that as the number of children in a family becomes greater, overcrowding adversely affects hygiene conditions, increasing the chance of contact with pathogens. There is also likely to be competition for the mother's attention and other resources.
The results from FGDs revealed that, due to participants' outdoors work, open defecation was common. This affects individuals' sanitation and is a cause of water source contamination. Where children play in the dirt, it may be contaminated by feces and act as a reservoir of pathogens. A further issue is that using treated water is not a common cultural practice in the society studied, increasing the likelihood of unsafe drinking water. Comprehensive diarrhoeal disease prevention is not easy in a poor community. Changing beliefs and habits, such as avoiding open defecation is difficult when there are no latrines close to markets, farms and other areas of work.
Strength and limitation
The strength of this study is that it was community based and specifically addressed acute childhood diarrhoeal morbidity in a rural community. Additionally, the study employed qualitative and quantitative methods, and had a high response rate (96.7%). However, the retrospective study design was a limitation. As this was a case-control study, there was potential for recall bias; however, this was minimized by using reported-incident cases within a 2 week period.
The determinants of acute childhood diarrhoea in this study were latrine availability, home-based water treatment, source of water and consumption of left-over food stored at room temperature. Also, a very high proportion of the adults interviewed defecated in open land, which can also increase the risk of diarrhoeal disease. The authors recommend the protection of water sources for the wellbeing of the community, and that the community is advised to treat drinking water to reduce the risk of diarrhoea among children. Food hygiene and general sanitation improvement is also highly recommended, as is further research into this vital area of child health.
Acknowledgments
The authors thank and acknowledge the Federal Ministry of Education of Ethiopia for funding this research. Special acknowledgement goes to Haramaya University for facilitating the funds from the Ministry of Education. The authors are deeply grateful to the data collectors and supervisors and the study community.
References
1. Kosek M, Bern C, Guerrant RL. The magnitude of the global burden of dirrhoeal disease from studies published 1992-2000. Bulletin of WHO 2003; 81: 197-204.
2. Pruss-Utun A, Kay D, Fewtrell L, Bartram J. Unsafe water, Sanitation and Hygiene. Comparative qualification of health risks. Geneva: WHO, 2004; 1321-1351.
3. Nyantekyi A, Mengistu L, Mulugeta B, Konjit T, Keberten M, Chanda M. Intestinal parasitic infections among under-five children and maternal awareness about the infections in Shesha Kekele, Wondo Genet, Southern Ethiopia. Ethiopian Journal of Health Development 2010; 24(3): 185-190.
4. Shimelis D. Effect of Zinc supplementation in treatment of acute diarrhoea among 2-59 months children treated in Black Lion Hospital, Addis Ababa, Ethiopia. Ethiopian Journal of Health Development. 2008; 22(2): 186-190.
5. USAID. Integrating sanitation and water supply programs. Annual Report in Africa. Washington DC: 2010.
6. Ahs JW, Wenjing T, Lofgren J, Forsberg BC. Diarrhoeal Diseases in Low- and Middle-Income Countries. Open Infectious Diseases Journal 2010; 4(123): 113-124.
7. Barreto ML, Genser B, Strina A, Teixeira MG, Assis AM, Rego RF et al. Effect of city-wide sanitation programme on reduction in rate of childhood diarrhoea in northeast Brazil: assessment by two cohort studies. Lancet 2007; 370: 1622-1628.
8. Wondwossen BA. Stepwise regression analysis on under-five diarrhael morbidity prevalence in Nekemte town, western Ethiopia: Maternal care giving and hygiene behavioral determinants. East African Journal of Public Health 2008; 5(3): 193-198.
9. Green S, Small J, Casman A. Determinants of National Diarrhoeal Disease Burden. Environmental Science & Technology 2009; 43(4): 123-131.
10. WHO/UNICEF. Why children are dying and what to be done? Geneva: WHO, 2009; 12-29.
11. Andualem A. Assessment of the impact of latrine utilization on diarrhoeal diseases in the rural community of Hulet Ejju Enessie Woreda, East Gojjam Zone, Amhara Region. Ethiopian Journal of Health Development. 2010; 24(3): 110-118.
12. Fewtrell L, Kaufmann B, Kay D, Enanoria W, Haller L, Colford J. Water, sanitation, and hygiene interventions to reduce diarrhoea in less developed countries. A systematic review and meta-analysis. Lancet Infectious Disease 2005; 5: 42-52.
13. Girma R, Wondwossen B, Bishaw D, Tefera B. Environmental determinants of diarrhoea among under-five children in Nekemte Town, western Ethiopia. Ethiopia Journal of Health Sciences 2007; 18(2): 39-44.
14. Tesfahun A, Mekasha A. Determinants of diarrhoeal diseases: a community based study in urban south western Ethiopia. East African Medical Journal 2003; 80(2): 77-82.
15. Vafaee A, Moradi A, Khabazkhoob M. Case-Control Study of acute diarrhoea in Children. Iran Journal Research and Health Sciences 2008; 8(1): 25-32.
16. Usman A, Rahaem A, Angola B. Exploring the Social and Environmental Determinants of Child Health in Ilorin, Nigeria. Ethiopian Journal of Environmental Studies and Management 2009; 2(3): 73-82.
17. Genser B, Strina A, Teles C, Prado M, Barreto M. Risk factors for acute diarrhoea incidence: dynamic analysis of a longitudinal study, Epidemiology; Salvador, Brazil. International Journal of Epidemiology 2006; 17(6): 658-667.
18. Takanashi K, Chonan Y, Quyen DT, Khan NC, Poudel KC, Jimba M. Survey of food-hygiene practices at home and childhood diarrhoea in Viet Nam. Journal of Health & Population Nutrition 2009; 27(5): 602-611.
19. Clasen T, Schmidt W, Rable T, Roberts I, Cairncross S. Interventions to improve water quality for preventing diarrhoea: Systematic review and meta-analysis. BMJ 2007; 334: 782-791.
20. Ehiri E, Ejemot I, Meremikwu M, Critchley A. Hand-washing for preventing diarrhoea. Cochrane Database of Systematic Reviews 2008; (1):CD004265.
21. Bettenheim M. The sanitation environment in urban slums: implications for child health, Population and Environment 2008; 30: 26-47.
22. Mediratta P, Amsalu F, Lawrence H, Sisay Y, Sack R. Risk Factors and Case Management of Acute Diarrhoea in North Gondar Zone, Ethiopia. Journal of Health Population and Nutrition 2010; 28(3): 253-263.
23. Teklu M. Socio-economic, environmental and behavioral factors associated with occurrence of diarrhoea among under-five in Maskenena Mareko Woreda, Southern Ethiopia. Master thesis, Addis Ababa University, 2003.


