Original Research
Relationship between COPD and lower socioeconomic status in farmers from south-eastern Poland (Lublin region)
AFFILIATIONS
1, 2, 6
Unit of Fibroproliferative Diseases, Institute of Rural Health, Lublin, Poland
3, 7
Department of Pneumonology, Oncology and Allergology, Medical University of Lublin, Lublin, Poland
4
Department of Zoonoses, Institute of Rural Health, Lublin, Poland
5
Department of Allergology and Environmental Hazards, Department of Internal Medicine and Occupational Diseases, Institute of Rural Health, Lublin, Poland
PUBLISHED
3 March 2014 Volume 14 Issue 1
HISTORY
RECEIVED: 11 February 2013
REVISED: 9 July 2013
ACCEPTED: 25 July 2013
CITATION
Golec M, Skórska C, Mackiewicz B, Dutkiewicz J, Góra A, Lemieszek M, Milanowski J. Relationship between COPD and lower socioeconomic status in farmers from south-eastern Poland (Lublin region). Rural and Remote Health 2014; 14: 2531. https://doi.org/10.22605/RRH2531
© Marcin Golec, Czesawa Skórska, Barbara Mackiewicz, Jacek Dutkiewicz, Anna Góra, Marta Lemieszek, Janusz Milanowski 2014 A licence to publish this material has been given to James Cook University, jcu.edu.au
Abstract
Introduction: Lower socioeconomic status is considered to be an independent risk factor of chronic conditions, such as chronic obstructive pulmonary disease (COPD). COPD, one of the major public health problems worldwide, is a chronic inflammatory lung disease of a multifactorial background. COPD morbidity in rural areas has been higher than in urban settings, as apart from the major causative factor, tobacco smoking, the burden of this disease in rural environments is also connected to additional occupational factors (organic dusts). The management of chronic diseases seems to be particularly difficult in rural areas. The aim of the study was to analyze the socioeconomic status of farmers suffering from COPD in comparison to healthy farmers.
Methods: Thirty farmers with COPD and 34 healthy farmers from the Lublin region (Poland) were investigated based on the area of land they possessed (an indicator used in the health insurance system in Poland to classify farmers). The farmers from five rural communes were selected by general practitioners. Statistical analysis was performed by non-parametric Mann-Whitney U-test for the differences between area of farms. The p<0.05 level was considered as significant.
Results: Area of land (median: 1.5 ha, 25th-75th percentile: 1.0-4.0) owned by farmers with COPD was significantly lower than area of farms belonging to healthy farmers (median: 7.0 ha, 25th-75th percentile: 3.0-10.0) (p<0.0001, Mann-Whitney U-test).
Conclusions: In rural areas individuals with COPD are characterized by significantly lower socioeconomic status than healthy persons. COPD is a major health problem, especially in rural areas, which may indicate that policy-makers should consider addressing equity in COPD management in rural areas.
Key words: COPD, equity, farmers, poverty, rural area.
Introduction
Chronic obstructive pulmonary disease (COPD) is one of the major, and still increasing, healthcare problems worldwide - causing considerable public health loss and increasing economic burden. The World Health Organization (WHO) defines COPD as 'a lung disease characterized by chronic obstruction of lung airflow that interferes with normal breathing and is not fully reversible'1. Diagnosis of COPD, besides presence of symptoms (like cough, sputum production, or dyspnoea) and physical examination, is mainly based on lung function testing (spirometry)1,2. Currently, COPD affects more than 200 million patients and has been predicted to become the third-leading cause of death within the next 20 years3,4. According to WHO, in Europe alone deaths from COPD are anticipated to rise by about 20%, from 248 000 in 2008 to about 300 000 in 20305-8. Given its increasing prevalence with age, and the inevitable ageing of societies globally, COPD probably poses one of the biggest threats for the world's healthcare systems.
Apart from the leading causative factor of COPD, which is tobacco smoking, other significant risk factors of this disease are cited by WHO, among them organic dusts (including biomass fuels and occupational dusts)9-11. According to the American Thoracic Society, in the USA occupational risk factors are responsible for about 20% of COPD cases in general (and, in case of non-smokers, this ratio goes up to about 30%)12,13. Indeed, occupational exposures, including organic dusts, are still an underestimated cause of COPD. This is particularly evident in relation to occupational biohazards affecting rural populations13,14. Millions of farmers and workers in the agricultural industry throughout the world are exposed to organic dust and thus the COPD morbidity in rural areas is usually higher than in urban areas15-34.
In Poland, no holistic epidemiological study, covering a representative sample of the Polish population, has been conducted concerning COPD35. In smaller studies COPD was identified in 10-26% of an examined group36-38. Respiratory diseases, led by the most frequently occurring one, COPD, are the fourth most common cause of death in Poland35,39. According to epidemiologic projections, the importance of these pathologies, including economic burden, will be increasing40.
The only study conducted in the Lublin region of Poland showed a high incidence of respiratory abnormalities of obstructive character in airways (11%) and a high ratio of patients from rural areas who did not receive proper treatment41. A study comparing rural and urban dwellers of the Krakow region supported the conclusion of higher prevalence of COPD in rural areas42.
Agricultural activity in Poland is still considerably higher than in many European countries (perhaps comparable only to countries such as France). Contrary to other Central and Eastern European countries, which also passed a period of Soviet domination, the land was never collectivized. The agricultural structure of small individual farms has prevailed43,44. Moreover, the decades under communist rule were characterized by increased fragmentation of the farm structure, leading to a growing number of small farms (1-10 ha). Even in 2005 the percentage of farms greater than 20 ha was only 4.5%45,46. Following this data, a number of farmers and inhabitants of rural areas is also considerable. A total of 16.2% of Polish inhabitants work as farmers. However, agriculture produces only 4.1% of Polish GDP47-50. Shrinking of agriculture share in Polish GDP caused by the economic transformation after 1989 was not followed by similarly significant adjustment in farmer numbers45,46,48. Only in the last few years has it started to bring a decline in the number of people employed on farms46,47.
Chronic conditions are strongly correlated with poverty. Socioeconomic status has been often described as an independent risk factor of a chronic condition51. This connection (also valid for COPD) has been thoroughly studied in developing countries52. However, growing evidence shows that in developed countries poverty and chronic diseases are also connected53-56. Lower socioeconomic status not only means lower quality of care but it also impairs prevention. It is more difficult for poor people to avoid the risk factor of a chronic disease, often due to lack of knowledge but also due to scarcity of resources57. This is especially difficult to address in rural areas as the management of chronic diseases, often requiring special policy tools, seems to be particularly difficult in remote places25,58. Rural areas are characterized by a significant distance to healthcare facilities, specialists, and by different epidemiologic features.
It is also worth mentioning that chronic diseases (including COPD) both in developed and developing countries used to affect mainly poorer parts of the population52-54. Additionally, COPD has an impoverishing effect on affected people59-63. In the rural area of Poland the issue of poverty connected to chronic diseases has been shown by Sygit et al64.
The aim of the study was to analyze the socioeconomic status of farmers suffering from COPD in comparison to healthy farmers.
Methods
Examined population
The presented work is a subject-based, cross-sectional study. A group of 30 farmers suffering from COPD (in stage I-II of the disease, according to GOLD (Global Initiative for Chronic Obstructive Lung Disease), an internationally recognized COPD classification), was examined2. The group comprised 17 men and 13 women, aged 54.3 ± 7.9 years (range 39-70 years). In the group 53.3% (16 persons) were tobacco smokers, 20% (6 persons) were ex-smokers, and 26.7% (8 persons) were non-smokers.
As a reference group 34 healthy farmers were examined. The group comprised 15 men and 19 women, aged 43.6 ± 13.1 years (range 20-68 years). In the group 23% (8 persons) were tobacco smokers, 12% (4 persons) were ex-smokers, and 65% (22 persons) were non-smokers.
The professionally active farmers - based on the diagnosis of COPD according to GOLD (stage I and II) and lack of other chronic illnesses for the first group, and lack of any chronic illness for the control group - were selected by their general practitioners from five rural communes in the Lublin region (south-eastern Poland): Fajsławice, Urzędów, Strzyżewice, Cyców and Kamionka (Fig1). The appropriateness of COPD diagnosis was additionally confirmed by a specialist of pulmonary medicine. (See 'Statistical analysis' below for results of statistical power calculation for sample sizes.)
Figure 1: Patients and healthy study participants recruitment: map of Lublin region and five rural communes.
Lung function testing
To confirm the diagnosis of COPD and stage of the COPD progression, each examination was conducted with the use of EasyOne Model 2001 spirometer (Medizintechnik AG, Zurich, Switzerland). Forced vital capacity (VC), forced expiratory volume in the first second (FEV1) and FEV1/VC (%) were measured. Both pre-shift and post-shift lung function examinations were conducted. Results were expressed as absolute values and as percentages of predicted values. The lung function testing was in accordance with European Respiratory Society guidelines65.
Questionnaire examination
The data (including the area of land owned by farmers) were gathered by using the questionnaire developed and validated at the Institute of Rural Health in Lublin for the examination of work-related symptoms caused by organic dusts66. In Poland, the criterion of owned area of land expressed in hectares determines the status of a farmer in relation to healthcare insurance67-69.
Statistical analysis
Statistical analysis was performed by non-parametric testing using the Mann-Whitney U-test for analysis of the differences between area of farms in both healthy and COPD groups. The p<0.05 level was considered as significant. As non-parametric tests were used, the data were mainly described by median and 25th-75th percentiles (except the description of lung function parameters which, traditionally, are described as mean ± standard deviation (SD)). All statistical analyses were carried out with Statistica v8.0 (Statsoft Inc.; http://www.statsoft.com).
Statistical power, calculated for measured values (area of land in hectares) and sample sizes (number of individuals in each of the two groups), was calculated as 99.9% (p<0.05 level). The DSS Statistical Power Calculator was used (two-tailed test) (DSS Research; http://www.dssresearch.com/KnowledgeCenter/toolkitcalculators.aspx).
Ethics approval
All subjects gave formal consent to participate in the study. The Ethics Commission of the Institute of Rural Health approved human subjects' protocols (Decision No. 11/2006).
Results
Lung function testing
The results of lung function assessment are shown in Table 1.
Land owned by farmers with COPD and by healthy farmers
Area of land owned by farmers with COPD (median: 1.5 hectares, 25th-75th percentile: 1.0-4.0 ha) was significantly lower than the area of farms belonging to healthy farmers (median: 7 ha, 25th-75th percentile: 3.0-10.0 ha) (p<0.0001, Mann-Whitney U-test). The results are shown in Figure 2.
Table 1: Lung function values in farmers with chronic obstructive pulmonary
disease (according to GOLD) and in healthy farmers. Values expressed
as mean ± standard deviation.

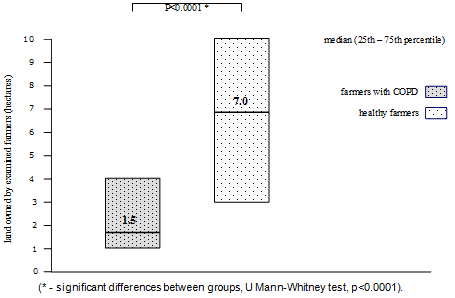 Figure 2: Area of farms owned by farmers with chronic obstructive pulmonary
Figure 2: Area of farms owned by farmers with chronic obstructive pulmonary
disease (COPD) and healthy farmers. (* Significant differences
between groups, Mann-Whitney U-test, p<0.0001).Discussion
Specific health issues of rural population have been neglected to a large extent both in the research literature as well as in the healthcare system design70. Indeed, only few studies tackle the issue of COPD management in rural areas or analyze its links to the plethora of risk factors, such as poverty, usually connected to chronic conditions. This is surprising given the number of studies and reviews indicating the significant scale of the problem of COPD in farmers as well as its specificity15-26. Characteristic occupational risk factors (such as organic dust exposure), followed by a prevalence of COPD have been well described in the literature18. Occupational factors are responsible for one-third of COPD cases in non-smokers12,13. The presented study addresses the issue of socioeconomic status in COPD in rural areas. It shows that farmers with COPD in the Lublin region (south-east Poland) may be characterized as having significantly lower socioeconomic status. As shown by the study, farmers with COPD possess significantly smaller pieces of land than healthy farmers (Fig2). However, the study results should be assessed with regards to some limitations, including heterogeneity between the COPD and healthy farmers' groups (eg lower age in the control group, compared to COPD individuals).
The results of the presented study, conducted in one of the regions of Poland, a country of moderate climate in Central Europe, with a quite developed agricultural sector, are in line with outcomes of identified studies about the role of poverty in COPD patients in rural areas71,72. Other studies show that poverty may be understood as an additional risk factor of chronic disease burden and as a cause determining other risk factors causing chronic conditions (eg tobacco smoking, the main source of COPD)57. Usually studies tackling the issue of poverty have been undertaken in low-income countries72; however, data from other high-income countries indicate that chronic conditions and poverty are combined also in rich countries53,54,56. Results of the study conducted in one of the most developed countries, Australia, reveal that poverty causes a decrease in quality of health services, even if these services are available73. Furler et al. postulate special means to increase quality of services in disadvantaged areas (like strengthening community health services, health promotion or removing financial disincentives concerning long consultations)73. According to these authors the number of general practitioners in disadvantaged rural areas should be increased to keep quality of care equal to that of wealthy areas.
Access to health services of proper quality is a result of a complex interaction between the patient on one side and the provider and system on the other. The ability to use health services of a sufficient amount and quality by a given group of patients (eg COPD patients in rural areas, belonging to poorer farmers) depends on economic resources and health literacy and certain attitudes (eg patient conceptualization of health care and seeking or negotiating help)74-77. Thus, in order to increase quality of healthcare delivery in disadvantaged communities, special health system design is needed. Different solutions are being proposed (eg co-location of services) so that disadvantaged patients have a chance to receive primary, social and specialist care in one location78.
The presence of competent, high-quality primary care seems to be the basis for proper COPD management. In rural areas79 this is not so simple as the engagement of general practitioners in remote locations may be difficult57. The role of competent nurses is also indicated80,81. Further technological development (eg IT, telemedicine) should bring at least a partial solution to these issues82.
In certain rural areas chronic respiratory illnesses are not connected to poverty: Huhti et al. in their study conducted in Finland do not see any differences in socioeconomic status and chronic respiratory conditions83.
Opinions about negative consequences of living in rural areas for patients with respiratory illnesses are though not shared by all authors. Iversen at al. show data of better respiratory health status of people from rural areas of Scotland than urban dwellers (lower prevalence of asthma and some respiratory conditions in countryside). However, Iversen et al. underline that COPD is excluded from this notice (according to the study prevalence of COPD is the same in rural and urban areas)84.
According to the authors' knowledge this is the first study analyzing the issue of socioeconomic status in rural areas concerning COPD in Poland. The results obtained from the current study suggest that COPD affects poorer farmers (a limitation of the study might be defining the farmers' socioeconomic status based only on one parameter: area of possessed land). Social inequalities are a recognized reason for worse health status in Poland, including the Lublin region85. The same is true for impairment of health services for poorer rural inhabitants64. If COPD is indeed connected to poverty in rural areas in Poland then the healthcare system should be re-designed to address this issue and deliver relevant quality of care to this population. In cases of COPD a low level of care results in additional hospital treatment for exacerbations and thus the total cost of COPD management per patient increases dramatically86,87.
Farmers with low socioeconomic status are taken care of in Poland: they are covered (basically for free) by the same coverage of health insurance as the rest of population. But health insurance coverage is not the only requirement to obtain sufficient healthcare services; there other issues: financial constraints, limited availability of providers, culturally inappropriate services (eg lack of IT literacy excludes some forms of care, eg telemonitoring)88. Authors analysing other diseases (eg cancer) note that patients from rural areas tend to use health services insufficiently, have worse outcomes and meet difficulties in accessing health services89,90. Probably due to its very nature (an ill person looking for services), the utilization of health services decreases with increasing distance of patients from healthcare facilities91. Addressing equity of health services for patients in rural, remote areas should be part of the scope of future reforms of modern healthcare systems92.
What is new for rural health? The study results show that, in Poland, COPD in rural areas affects the poorer parts of the population. Practical implications of the study outcome might be attempts to improve working conditions in smaller farms (lower exposure to organic dusts and other harmful occupational factors causing COPD) and paying special attention to equity of access while dealing with COPD management in rural areas.
Conclusions
In rural areas individuals with COPD have a significantly lower socioeconomic status than healthy persons. Given that COPD is a major health problem, especially in rural areas, this may indicate that policy-makers should consider addressing equity in COPD management in rural areas.
Acknowledgements
Examinations were part of two government projects funded respectively by the Ministry of Science and Higher Education and the Ministry of Research and Technology, Republic of Poland: 'Role of the peptide LL-37 in pathogenesis of the chronic obstructive pulmonary disease' (project no. N402 056 32/1659); 'Occupational exposure to dust from herbs in agriculture' (project no. 3 PO5D 081 24).
References
1. World Health Organization. Chronic respiratory diseases, COPD. Online (no date). Available: http://www.who.int/respiratory/copd/definition/en/index.html (Accessed 7 April 2012).
2. Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, et al. Global Initiative for Chronic Obstructive Lung Disease: Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. American Journal of Respiratory and Critical Care Medicine 2007; 176: 532-555.
3. Calderón-Larrañaga A, Carney L, Soljak M, Bottle A, Partridge M, Bell D, et al. Association of population and primary healthcare factors with hospital admission rates for chronic obstructive pulmonary disease in England: national cross-sectional study. Thorax 2011; 66: 191-196.
4. World Health Organization. COPD burden. (Online) 2010. Available: http://www.who.int/respiratory/copd/burden/en (Accessed 1 September 2012).
5. World Health Organization. Chronic diseases and health promotion. Online (no date). Available: http://www.who.int/chp/chronic_disease_report/part2_ch2/en/index.html (Accessed 20 November 2011).
6. WHO. Noncommunicable diseases country profiles 2011. (Online) 2011. Available:http://whqlibdoc.who.int/publications/2011/9789241502283_eng.pdf (Accessed 20 November 2012).
7. WHO. 2008-2013 Action Plan for the Global Strategy for the Prevention and Control of Noncommunicable Diseases. Available: http://whqlibdoc.who.int/publications/2009/9789241597418_eng.pdf (Accessed 20 November 2012).
8. WHO. Global status report on noncommunicable diseases 2010. Geneva: WHO 2010. Available: http://whqlibdoc.who.int/publications/2011/9789240686458_eng.pdf, (Accessed 20 November 2012).
9. WHO. Chronic respiratory diseases, causes of COPD. Available: http://www.who.int/respiratory/copd/causes/en/index.html (Accessed 1 September 2012).
10. Pauwels RA, Rabe KF. Burden and clinical features of chronic obstructive pulmonary disease (COPD). Lancet 2004; 364(9434): 613-620.
11. Viegi G, Pistelli F, Sherrill DL, Maio S, Baldacci S, Carrozzi L. Definition, epidemiology and natural history of COPD. European Respiratory Journal 2007; 30: 993-1013.
12. Balmes J, Becklake M, Blanc P, Henneberger P, Kreiss K, Mapp C, et al. Environmental and Occupational Health Assembly, American Thoracic Society. American Thoracic Society Statement: Occupational contribution to the burden of airway disease. American Journal of Respiratory and Critical Care Medicine 2003; 167: 787-797.
13. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (revised 2011). In: Global Initiative for Chronic Obstructive Lung Disease. (Online) 2011. Available: http://www.goldcopd.org/Guidelines/guidelines-resources.html (Accessed 7 April 2012).
14. Matheson MC, Benke G, Raven J, Sim MR, Kromhout H, Vermeulen R, et al. Biological dust exposure in the workplace is a risk factor for chronic obstructive pulmonary disease. Thorax 2005; 60: 645-651.
15. Bailey KL, Meza JL, Smith LM, Von Essen SG, Romberger DJ. Agricultural exposures in patients with COPD in health systems serving rural areas. Journal of Agromedicine 2007, 12: 71-76.
16. Danuser B, Weber C, Künzli N, Schindler C, Nowak D. Respiratory symptoms in Swiss farmers: an epidemiological study of risk factors. American Journal of Industrial Medicine 2001; 39: 410-418.
17. do Pico GA. Hazardous exposure and lung disease among farm workers. Clinics in Chest Medicine 1992; 13(2): 311-328.
18. Eduard W, Pearce N, Douwes J. Chronic bronchitis, COPD, and lung function in farmers: the role of biological agents. Chest 2009; 136: 716-725.
19. Greskevitch M, Kullman G, Bang KM, Mazurek JM. Respiratory disease in agricultural workers: mortality and morbidity statistics. Journal of Agromedicine (2007); 12(3): 5-10.
20. Idolor LF, de Guia TS, Francisco NA, Roa CC, Ayuyao FG, Tady CZ, et al. Burden of obstructive lung disease in a rural setting in the Philippines. Respirology 2011; 16(7): 1111-1118.
21. Jouneau S, Boché A, Brinchault G, Fekete K, Guillot S, Bayat S, et al. On-site screening of farming-induced chronic obstructive pulmonary disease with the use of an electronic mini-spirometer: results of a pilot study in Brittany, France. International Archives of Occupational and Environmental Health 2012; 85(6): 623-630.
22. Kroneman M, Verheij R, Tacken M, van der Zee J. Urban-rural health differences: primary care data and self reported data render different results. Health Place 2010; 16(5): 893-902.
23. Lâm HT, Rönmark E, Tu'ò'ng NV, Ekerljung L, Chúc NT, Lundbäck B. Increase in asthma and a high prevalence of bronchitis: results from a population study among adults in urban and rural Vietnam. Respiratory Medicine 2011; 105(2): 177-185.
24. Linaker C, Smedley J. Respiratory illness in agricultural workers. Occupational Medicine (London) 2002; 52(8): 451-459.
25. Omland Ø. Exposure and respiratory health in farming in temperate zones - a review of the literature. Annals of Agricultural and Environmental Medicine 2002; 9: 119-136.
26. O'Reilly G, O'Reilly D, Rosato M, Connolly S. Urban and rural variations in morbidity and mortality in Northern Ireland. BMC Public Health 2007; 7: 123.
27. Pandey MR. Prevalence of chronic bronchitis in a rural community of the Hill Region of Nepal. Thorax 1984; 39: 331-336.
28. Po JY, FitzGerald JM, Carlsten C. Respiratory disease associated with solid biomass fuel exposure in rural women and children: systematic review and meta-analysis. Thorax 2011; 66: 232-239.
29. Radon K, Garz S, Riess A, Koops F, Monso E, Weber C, et al. European Farmers' Project. Respiratory diseases in European farmers-II. Part of the European farmers' project. Pneumologie 2003; 57: 510-517.
30. Rautiainen RH, Reynolds SJ. Mortality and morbidity in agriculture in the United States. Journal of Agricultural Safety and Health 2002, 8(3): 259-276.
31. Szczyrek M, Krawczyk P, Milanowski J, Jastrzębska I, Zwolak A, Daniluk J. Chronic obstructive pulmonary disease in farmers and agricultural workers - an overview. Annals of Agricultural and Environmental Medicine 2011; 18(2): 310-313.
32. von Essen SG, Banks DE. Life-long exposures on the farm, respiratory symptoms, and lung function decline. Chest 2009; 136(3): 662-663.
33. Xu F, Yin X, Zhang M, Shen H, Lu L, Xu Y. Prevalence of physician-diagnosed COPD and its association with smoking among urban and rural residents in regional mainland China. Chest 2005; 128(4): 2818-2823.
34. Zhong N, Wang C, Yao W, Chen P, Kang J, Huang S, et al. Prevalence of chronic obstructive pulmonary disease in china: a large, population based survey. American Journal of Respiratory and Critical Care Medicine 2007; 176(8): 753-760.
35. Pierzchała W, Barczyk A, Górecka D, Sliwiński P, Zieliński J. Polish Society of Lung Diseases. Recommendations of Polish Society of Lung Diseases about diagnosis and therapy of chronic obstructive pulmonary disease (COPD). Pneumonologia i Alergologia Polska 2010; 78(5): 318-347.
36. Niepsuj G, Kozielski J, Niepsuj K. Przewlekła obturacyjna choroba płuc wśród mieszkańców Zabrza. Wiadomości Lekarskie 2002; 55 (suppl. 1): 354-359.
37. Niżankowska-Mogilnicka E, Mejza F, Buist AS Vollmer WM, Skucha W, Harat R, Pajak A, et al. Prevalence of COPD and tobacco smoking in Malopolska region - results from the BOLD study in Poland. Polskie Archiwum Medycyny Wewnętrznej 2007; 117: 402-410.
38. Bednarek M, Maciejewski J, Wozniak M, Kuca P, Zielinski J. Prevalence, severity and underdiagnosis of COPD in the primary care setting. Thorax 2008; 63(5): 402-407.
39. Central Statistical Office (Główny Urząd Statystyczny, GUS). Statistical yearbook 2008. Warsaw, Poland, 2008.
40. Poznańska A, Wojtyniak B, Seroka W. Main causes of death among Polish population in 2030. Przegląd Epidemiologiczny 2011; 65(3): 483-489.
41. Paprzycki P, Panasiuk L, Sodolski W. Prevalence of obstructive respiratory disorders in the rural population of the Lublin region. Annales Universitatis Mariae Curie Skłodowska Sectio D Medicina 2003; 58(1): 72-78.
42. Krawczyk K, Skucha W. Incidence of chronic obstructive pulmonary disease among chronic smokers inhabiting Krakow and Proszowice. Przegląd Lekarski 2000; 57(11): 617-618.
43. OECD. Rural development. In: OECD Economic Surveys 2004 Poland. (Online) 2004. Available : http://.oecd.org/eco (Accessed 15 December 2012); 164-189.
44. OECD. The Agri-environmental Situation and Policies in the Czech Republic, Hungary and Poland. (Online) 1999. Available: http://.oecd.org/tad/env (Accessed 15 December 2012).
45. Central Statistical Office (Główny Urząd Statystyczny, GUS). Statistical yearbook of agriculture and rural areas 2006. (Online) 2006. Available: http://.stat.gov.pl/ (Accessed 15 December 2012).
46. Latruffe L, Balcombe K, Davidova S, Zawalińska K. Determinants of technical efficiency of crop and livestock farms in Poland. Applied Economics 2004; 36: 1255-1263.
47. OECD. Environmental performance of agriculture in OECD countries since 1990. Paris: OECD, 2008.
48. Ministry of Agriculture and Rural Development. National strategic plan for 2007-2013 rural development. (Online) 2006. Available: http://.minrol.gov.pl/DesktopDefault.aspx?TabOrgId=1210&LangId=1 (Accessed 15 December 2012).
49. Ministry of Agriculture and Rural Development. Agriculture and food economy in Poland 2004-2006. (Online) 2006. Available: http://.minrol.gov.pl/DesktopDefault.aspx?TabOrgId=1210&LangId=1 (Accessed 15 December 2012).
50. Ministry of Agriculture and Rural Development. Rural development plan for poland 2004-2006. (Online) 2005. Available: http://.minrol.gov.pl/DesktopDefault.aspx?TabOrgId=1210&LangId=1 (Accessed 15 December 2012).
51. Yin P, Zhang M, Li Y, Jiang Y, Zhao W. Prevalence of COPD and its association with socioeconomic status in China: findings from China Chronic Disease Risk Factor Surveillance 2007. BMC Public Health 11: 586. (Online) 2011. Available http://www.biomedcentral.com/1471-2458/11/586 (Accessed 15 December 2012).
52. Aït-Khaled N, Enarson D, Bousquet J. Chronic respiratory diseases in developing countries: the burden and strategies for prevention and management. Bulletin of the World Health Organization 2001; 79(10): 971-979.
53. Colley JRT, Reid DD. Urban and social origins of childhood bronchitis in England and Wales. British Medical Journal 1970; 2: 213-217.
54. Prescott E, Lange P, Vestbo J. Socioeconomic status, lung function and admission to hospital for COPD: results from the Copenhagen City Heart Study. European Respiratory Journal 1999; 13(5): 1109-1114.
55. Busse R, Blümel M, Scheller-Kreinsen D, Zentner A. Tackling chronic disease in Europe. Strategies, interventions and challenges. Copenhagen: World Health Organization, on behalf of the European Observatory on Health Systems and Policies, 2010.
56. Suhrcke M, Urban D. Are cardiovascular diseases bad for economic growth? Copenhagen: WHO Regional Office for Europe, 2006.
57. Nolte E, Mekce M. Managing chronic conditions. Experience in eight countries. WHO on behalf of the European Observatory on Health Systems and Policies. Maidenhead Berkshire, England: Open University Press, McGraw-Hill Education, 2008.
58. Abrams TE, Vaughan-Sarrazin M, Fan VS, Kaboli PJ. Geographic isolation and the risk for chronic obstructive pulmonary disease-related mortality: a cohort study. Annals of Internal Medicine 2011; 155: 80-86.
59. Aït-Khaled N, Enarson DA, Chiang CY. COPD management. Part II. Relevance for resource-poor settings. International Journal of Tuberculosis and Lung Diseases 2008; 12(6): 595-600.
60. Bergofsky L, Barron E, Goodwin RE. Putting the poor first. A system's assessment project identifies community needs. Health Programs 1991; 72(10): 64-67.
61. Gershon AS, Warner L, Cascagnette P, Victor JC, To T. Lifetime risk of developing chronic obstructive pulmonary disease: a longitudinal population study. Lancet 2011; 378(9795): 991-996.
62. Priest J, Buikema A, Engel-Nitz NM, Cook CL, Cantrell CR. Quality of care, health care costs, and utilization among Medicare Part D enrollees with and without low-income subsidy. Population Health Management 2012; 15(2): 101-112.
63. Sousa RM, Ferri CP, Acosta D, Albanese E, Guerra M, Huang Y, et al. Contribution of chronic diseases to disability in elderly people in countries with low and middle incomes: a 10/66 Dementia Research Group population-based survey. Lancet 2009; 374(9704): 1821-1830.
64. 64. Sygit K, Kołłątaj W, Sygit M, Kołłątaj B. The impact of economic factors on the realities of outpatient multi-drug treatment of chronic diseases in rural areas. Annals of Agricultural and Environmental Medicine 2011; 18: 29-34.
65. Quanjer PH, Tammeling GJ, Cotes JE, Pedersen OF, Peslin R, Yernault JC. Lung volumes and forced ventilatory flows. Report, Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official statement of the European Respiratory Society. European Respiratory Journal 1993; 16: 5-40.
66. Dutkiewicz J, Mackiewicz B. Kwestionariusz opracowany w Instytucie Medycyny Wsi w Lublinie dotyczący ekspozycji na pyły organiczne i wywoływanych przez nie objawów (Annex) (The questionnaire created in the Institute of Rural Health for studying exposure to organic dusts and work related symptoms). In: J Dutkiewicz, C Skórska, B Mackiewicz, G Cholewa (Eds). Zapobieganie Chorobom Wywoływanym przez Pyły Organiczne w Rolnictwie i Przemyśle Rolnym (in Polish). Lublin: Institute of Rural Health, 2000; 85-88.
67. Agricultural Social Insurance Fund (KRUS). Available http://www.krus.gov.pl/en. (Accessed 17 December 2012).
68. Polish Official Journal. 2012 Farmers' Health Insurance Act. Official Journal, item 123, 2012.
69. Polish Official Journal. The Act of 20 December 1990 on Farmers' Social Insurance. Full Text with Later Amendments. Official Journal No. 50 of 2008, item 291, 2008.
70. Effing T, Monninkhof EM, van der Valk PD, van der Palen J, van Herwaarden CLA, Partridge MR, et al. Self-management education for patients with chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2007; 4: 1-398.
71. Deveci F, Deveci SE, Türkoğlu S, Turgut T, Kirkil G, Rahman Set al. The prevalence of chronic obstructive pulmonary disease in Elazig, Eastern Turkey. European Journal of Internal Medicine 2011; 22(2): 172-176.
72. Fullerton DG, Suseno A, Semple S, Kalambo F, Malamba R, White S, et al. Wood smoke exposure, poverty and impaired lung function in Malawian adults. International Journal of Tuberculosis and Lung Diseases 2011; 15(3): 391-398.
73. Furler JS, Harris E, Chondros P, Powell Davies PG, Harris MF, Young DY. The inverse care law revisited: impact of disadvantaged location on accessing longer GP consultation times. Medical Journal of Australia 2002; 177(2): 80-83.
74. Whitehead M. The Concepts and Principles of Health and Equity. Copenhagen: World Health Organization Europe, 1990.
75. Penchansky R, Thomas JW. The concept of access. Definition and relationship to consumer satisfaction. Medical Care 1981; 19(2): 127-140.
76. Rogers A, Flowers J, Pencheon D. Improving access needs a whole systems approach. British Medical Journal 1999; 7214: 866-867.
77. Harris MF, Furler JS, Mercer SW, Willems SJ. Equity of access to quality of care in family medicine. International Journal of Family Medicine (Online) 2011; doi: 10.1155/2011/858131 (Accessed 15 December 2012).
78. Marmot M. Social determinants of health - what doctors can do. London: British Medical Association, 2011.
79. Probst JC, Laditka JN, Laditka SB. Association between community health center and rural health clinic presence and county-level hospitalization rates for ambulatory care sensitive conditions: an analysis across eight US states. BMC Health Services Research 2009; 9: 134, doi: 10.1186/1472-6963-9-134.
80. Hopley M, Horsburgh M, Peri K. Barriers to accessing specialist care for older people with chronic obstructive pulmonary disease in rural New Zealand. Journal of Primary Health Care 2009; 1(3): 207-214.
81. Canadian Institute for Health Information. How healthy are rural Canadians? An assessment of their health status and health determinants. Ottawa, ON: Government of Canada, 2006. http://www.phac-aspc.gc.ca/publicat/rural06/ (Accessed 15 December 2012).
82. Stellefson M, Chaney BH, Chaney JD. Using exploratory focus groups to inform the development of targeted COPD self-management education DVDs for rural patients. International Journal of Telemedicine and Applications 2010; doi: 10.1155/2010/450418.
83. Huhti E, Takala J, Nuutinen J, Poukkula A. Chronic respiratory disease in rural women. An epidemiological survey at Hankasalmi, Finland. Annals of Clinical Research 1978; 10(2): 95-101.
84. Iversen L, Hannaford PC, Price DB, Godden DJ. Is living in a rural area good for your respiratory health? Results from a cross-sectional study in Scotland. Chest 2005; 128(4): 2059-2067.
85. Kołodziej H, Łopuszańska M. Regional differences in premature mortality in Poland. Przegląd Epidemiologiczny 2010; 64(4): 543-550.
86. Jahnz-Różyk K, Targowski T, From S, Faluta T, Borowiec L. Costs of chronic obstructive pulmonary disease in patients treated in ambulatory care in Poland. Pneumonologia i Alergologia Polska 2011; 79(5): 337-342.
87. Jahnz-Rózyk K, Targowski T. Costs of exacerbations of chronic obstructive pulmonary disease in primary and secondary care in 2007 - results of multicenter Polish study. Polski Merkuriusz Lekarski 2009; 26(153): 208-214.
88. Mayberry RM, Nicewander DA, Qin H, Ballard DJ. Improving quality and reducing inequities: a challenge in achieving best care. Proceedings (Baylor University Medical Centre) 2006; 19(2): 103-118.
89. Bain NSC, Campbell NC. Treating patients with colorectal cancer in rural and urban areas: a qualitative study of the patients' perspective. Family Practice 2000; 17: 475-479.
90. Goyder EC, Blank L, Ellis E, Furber A, Peters J, Sartain K, Massey C. Reducing inequalities in access to health care: developing a toolkit through action research. Quality and Safety in Health Care 2005; 14(5): 336-339.
91. Mungall IJ. Trend towards centralization of hospital services and its effect on access to care for rural and remote communities in the UK. Rural and Remote Health 5: 390. (Online) 2005. Available: www.rrh.org.au (Accessed 3 September 2012).
92. Kenealy TW, Connolly MJ, Mahony F, Barber PA, Boyd MA, Carswell P, et al. Health equity in the New Zealand health care system: a national survey. International Journal of Equity in Health 2011; 10: 45.



