The prevalence of chronic liver disease (CLD) in the Aboriginal North American population is disproportionately higher than that of the non-indigenous population. Hepatitis C virus (HCV) is the second leading cause of CLD in American Indians or Alaska Natives (AIANs), with a mortality rate of 2.3 per 100 000 compared to 1.7 per 100 000 in the general US population1,2.
Emerging data strongly suggest that AIAN ethnicity influences the outcome of antiviral treatment to HCV due to genetic variability in production of pro-inflammatory cytokines in response to interferon-gamma2-4. However, population-based studies demonstrating outcome variability for HCV treatment comparing AIAN to other ethnicities are scarce. In North Dakota, about 5% of its more than 670 000 population in 2010 identified themselves as AIAN. More than 50% of them were living in reservations5. The purpose of this study is to describe the experience of a North Dakota teaching community hospital and Veterans Affairs Medical Center in treating HCV among AIANs and to compare treatment outcomes to a cohort of Caucasian patients.
Study design
This is a retrospective, multi-center, descriptive study involving AIANs with a diagnosis of HCV from June 2008 to December 2011 at Sanford Health North and Fargo Veterans Affairs Medical Center in North Dakota. A comparison of response to treatment was done between those who received a combination of pegylated-interferon and ribavirin and a random group of Caucasians.
Patients
Patients included in this study were adults aged 18 years or older with chronic HCV infection confirmed by a quantitative test for HCV ribonucleic acid (RNA) that received combination antiviral treatment.
The treating gastroenterologists determined treatment eligibility, and typical exclusion criteria were concomitant liver disease, decompensated cirrhosis (Child-Torcotte-Pugh score >6), neutropenia (white blood cell count <1500/mL) and/or thrombocytopenia (platelets <100 000/mL), end-stage renal disease, active psychiatric diseases, active alcohol or other substance abuse, severe cardiopulmonary diseases or pregnancy. Both interferon-naive and previously treated patients were eligible.
Demographics of the patient population were recorded including ethnicity, age, sex, body mass index (BMI), risk factor for contracting hepatitis C, co-infection with HIV or hepatitis B, HCV genotype, alanine aminotransferase (ALT) and viral load at presentation and biopsy stage (if available). Also recorded were patients' HCV treatment status in the past (treatment naivety). Aspartate aminotransferase (AST) platelet ratio index (APRI score), the ratio of AST and platelets obtained within 6 months prior to biopsy were calculated and recorded for all patients who underwent liver biopsy6.
Assessments and outcomes
The study describes characteristics of AIANs in North Dakota diagnosed with HCV. The proportion of patients who received treatment is assessed. Documented reasons for not receiving treatment are analyzed.
A comparison of AIANs who received treatment was also done with a random group of Caucasians. Primary endpoint was sustained virological response (SVR) defined by undetectable HCV-RNA viral load by qualitative polymerase chain reaction (PCR) at or after 6 months of completing treatment, regardless of treatment duration. Since early response to treatment was found to be independent predictors for treatment outcome in previous studies, HCV RNA viral load was recorded for all patients who received treatment at week 4 (if available), week 12 and end of treatment (regardless of treatment length)7.
A rapid virological response (RVR) at week 4, and early virological response (EVR) at week 12 were defined as undetectable HCV-RNA by qualitative PCR, or a 2log10 reduction or more in HCV-RNA relative to the baseline value by quantitative PCR.
For all patients who received treatment, data was collected on treatment length, continuation or discontinuation of treatment, reasons for discontinuation, and adverse events.
Statistical analysis method
Mean and median values were computed for all continuous variables, and frequency distributions were calculated for all categorical variables. We compared AIANs to Caucasian patients, all of whom were diagnosed with hepatitis C on demographic and other variables using Wilcoxon signed-rank test for non-normally distributed continuous variables and χ2 or Fisher's exact tests for categorical variables. We also compared AIANs who received treatment with untreated AIANs. All statistical tests were two-tailed with p<0.05 considered to be significant. Statistics were performed using SAS v9.3 (SAS Institute, http://www.sas.com/en_us/home.html).
Ethics approval
The study was approved by the department scholarly activity committee and received exemption from the institutional review board.
A total of 124 adult patients with HCV infection self-identified as AIANs. Only 22 (18%) of them received treatment. The most common reason for not receiving treatment was inability to make appointments or lack of referral to a specialist 37 (36%). Other identified factors included low or undetectable viral load (n=22 (22%)), concomitant or decompensated liver disease (n=21 (20%)), alcohol and drug abuse (n=17 (17%)) and cost of treatment (n=2 (2%)).
Table 1 shows the demographics and clinical characteristics of AIANs and a random group of 53 Caucasians diagnosed with HCV that received combination antiviral treatment. There were no significant differences in the baseline characteristics between these two groups. The key predictors of SVR, including distribution of genotypes, high viral load, ALT and advanced fibrosis stages, were not statistically different in AIANs compared to Caucasian controls.
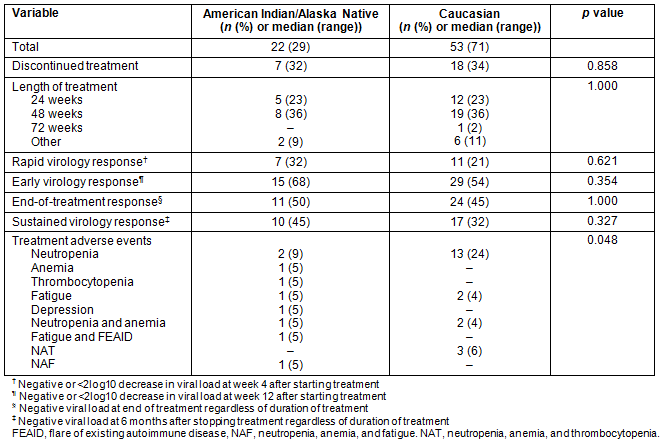
Of the 22 AIAN patients who received treatment, only 15 completed the course. Of those who discontinued treatment, four patients discontinued because of side effects, two had non-response at week 12 and one was lost to follow-up. Among Caucasian patients, 35 out of 53 completed therapy. Table 2 describes the treatment responses and adverse effects of treatment between the two groups. Among patients for whom a week 12 HCV-RNA assay result was reported (18 AIANs, 41 Caucasians), an EVR was achieved in 15 (83%) and 29 (77%) of AIANs and Caucasians respectively. An end-of-treatment virological response was achieved in 11 (50%) of AIANs versus 24 (45%) of Caucasian controls who completed therapy. The overall SVR rate was higher in AIANs (n=10 (45%)) compared to 17 (32%) of controls, although not statistically significant.
Table 1: Demographic and clinical characteristics of patients diagnosed with hepatitis C, by ethnicity (n=75)
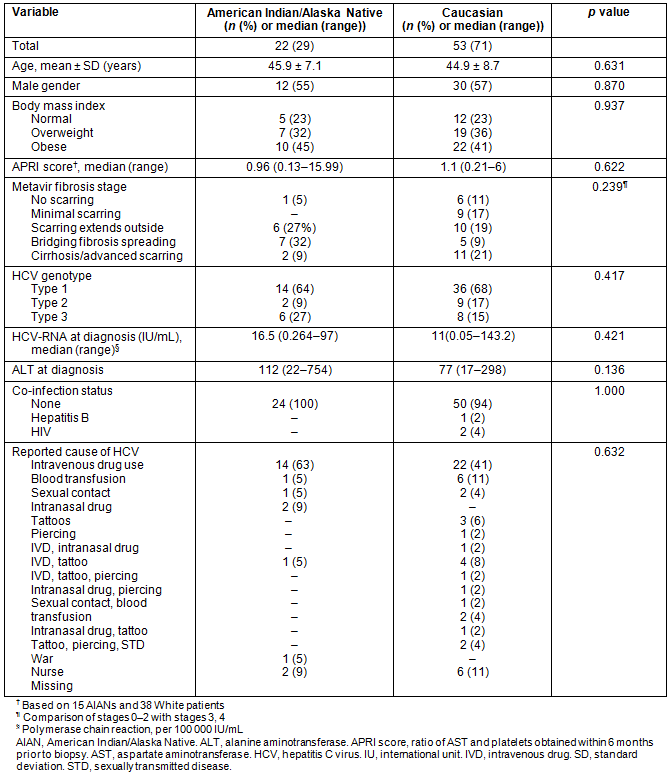
Table 2: Treatment response and side effects of patients diagnosed with hepatitis C, by ethnicity (n=75)
Discussion
Several studies suggested that a HCV treatment in AIANs may have more favorable outcomes2. This may be based on higher prevalence of more favorable genotypes but also may be related to distinct Aboriginal cytokine profiles that favor interferon-gamma over interleukin-10 synthesis relative to Caucasian cells. These were seen to potentially promote enhanced viral clearance in Aboriginal populations8.
Multiple dynamics contribute to the racial differences in acquisition risk factors, access and response to treatment of HCV infection between AIANs and Caucasians9. In our study, we found that only 18% of AIAN patients diagnosed with HCV infection received treatment. Inability to show up to appointments or lack of referrals was noted to be the biggest hurdle in getting treatment. Specialty clinics and gastroenterologists were only present in the urban areas and not in AIAN communities. Other personal and community factors may prevent these patients from making the clinic visits. Rempel and Uhanova suitably referred to HCV infection as part of the 'broken spirit' diseases. Historical and personal trauma influence participation in high risk behaviors such as intravenous drug use which is a major risk factor for acquisition10. Health policy makers should take these factors in consideration for an intervention program to be successful.
In addressing specialty healthcare access limitations, teleconference or Extensions for Community HealthCare Outcomes program may be a useful way to reach out to these populations. This allows follow up visits for patients who would otherwise not be able to see their providers if travel is an issue. It provides remote delivery of healthcare services through the use of telecommunications technology.
Despite or possibly due to the small number of AIAN population who received treatment, we were unable to find any significant differences in treatment responses between AIANs and Caucasians. However, adverse events were less, especially neutropenia, among AIANs.
Only a few studies have compared the outcome of interferon or interferon-ribavirin therapy in AIANs compared to non-AIAN groups2,11. The Pegasys trial in Canada showed there was no significant difference in sustained virological response in the two groups2 whereas the Alaska Native Tribal Health Consortium Hepatitis Research Program reported low SVR rates in a longitudinal study of Alaskan natives11. Full understanding of potential differences supports the need for tailored therapy based on racial differences. Published data suggests highest response rate in Asians, followed by Caucasians, but data is scarce for AIAN patients12,13. Our random control group consisted of Caucasians because they constitute most of our general patient population in this part of the USA.
Alhough not statistically significant, a trend of higher viral load, ALT and fibrosis score in AIAN patients compared to the control population was reported. This may be explained by lag time in referral, low uptake of care and/or reluctance in this population to follow up.
The present study has several limitations. It was only conducted in two medical institutions and our sample size is small. It has the inherent limitations of a retrospective study and relies on documentation on the chart which constrains our full understanding of certain parameters such as personal and social factors influencing access and perceptions to care. This is a good direction for future research in this population. A possible goal would be to fully understand personal and community barriers to evaluation and treatment. In North Dakota, the epidemiological profile of liver diseases is continuously surveyed as part of the Department of Health's Viral Hepatitis Program, the mission of which is to protect the health of the public by prevention and control of viral hepatitis infection. In 2011, 11% of patients with HCV infection were identified as AIANs. However, a deeper understanding of the community may be needed to shed light into the statistics and focus resources for policies and interventions that would be most beneficial.
We found that most of AIANs with HCV infection do not receive treatment. The most common reasons were lack of follow-up or referral to a specialist, concomitant or decompensated liver disease and polysubstance abuse. Response to treatment showed no significant difference among AIANs compared to Caucasians, a population reported to have a relatively favorable response to treatment. This study does not contradict laboratory research data suggesting enhanced virological clearance in Aboriginals. Further population-based studies are warranted, because it is crucial to treat the chronic HCV infection to decrease the burden of disease in the AIAN community. It may be of value to consider optimization of medical comorbidities and ensure increased uptake of care or implement aggressive follow-up measures in order to enroll more AIAN population for treatment.
References
1. Scott JD, Garland N. Chronic liver disease in Aboriginal North Americans. World Journal of Gastroenterology 2008; 14(29): 4607-4615.
2. Cooper CL, Bailey RJ, Bain VG, Anderson F, Yoshida EM, Krajden M, et al. Outcomes of peginterferon alpha-2a and ribavirin hepatitis C therapy in Aboriginal Canadians. Canadian Journal of Gastroenterology 2008; 22(8): 677-680.
3. Aborsangaya KB, Dembinski I, Khatkar S, Alphonse MP, Nickerson P, Rempel JD. Impact of Aboriginal ethnicity on HCV core-induced IL-10 synthesis: interaction with IL-10 gene polymorphisms. Hepatology 2007; 45(3): 623-630.
4. Rempel JD, Aborsangaya KB, Alphonse MP, Minuk GY. The influence of North American Aboriginal ethnicity on pro?inflammatory and anti?inflammatory cytokine responses to IFN?alpha. Journal of Viral Hepatology, 2009; 16(4): 292-297.
5. North Dakota Indian Affairs Commission. (Online) 2010. Available: http://www.nd.gov/indianaffairs/?id=37. (Accessed 13 November 2013).
6. Lin ZH, Xin YN, Dong QJ, Wang Q, Jiang XJ, Zhan SH, et al. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: an updated meta-analysis. Hepatology 2011; 53(3): 726-736.
7. Martinot-Peignoux M, Maylin S, Moucari R, Ripault MP, Boyer N, Cardoso AC, et al. Virological response at 4 weeks to predict outcome of hepatitis C treatment with pegylated interferon and ribavirin. Antiviral Therapy 2009; 14(4): 501-511.
8. McMahon BJ, Hennessy TW, Christensen C, Bruden D, Sullivan DG, Homan C, et al. Epidemiology and risk factors for hepatitis C in Alaska Natives. Hepatology 2004; 39(2): 325-332.
9. Wu HX, Wu J, Wong T, Andonov A, Li Q, Dinner K, et al. Incidence and risk factors for newly acquired hepatitis C virus infection among Aboriginal versus non-Aboriginal Canadians in six regions, 1999-2004. European Journal of Clinical Microbiology and Infectious Diseases 2007; 26(3): 167-174.
10. Rempel JD, Uhanova J. Hepatitis C virus in American Indian/Alaskan Native and Aboriginal Peoples of North America. Viruses 2012; 4(12): 3912-3931.
11. Christensen C, Bruden D, Livingston S, Williams J, Homan C, Sullivan D, et al. Hepatitis C treatment results in an Alaska native/American Indian population. Hepatology 2007; 42(4 Suppl 1): 654A-655A.
12. Muir AJ, Hu KQ, Gordon SC, Koury K, Boparai N, Noviello S, et.al. Hepatitis C treatment among racial and ethnic groups in the IDEAL trial. Journal of Viral Hepatology 2011; 18(4): e134-e143.
13. Hu KQ, Freilich B, Brown RS, Brass C, Jacobson IM. Impact of Hispanic or Asian ethnicity on the treatment outcomes of chronic hepatitis C: results from the WIN-R trial. Journal of Clinical Gastroenterology 2011; 45(8): 720-726.
