Differential patterns of cancer incidence and mortality have been observed between rural and urban communities around the world1-3. Social, economic, cultural, occupational and environmental factors have been suggested as the major driving forces behind these disparities4-7. One of the most plausible hypotheses is the potential link between social deprivation and rurality, which is associated with decreased access to health care and specialized treatment8-10. This discrepancy is realized both in terms of secondary prevention (early cancer screening), as well as at the level of advanced therapeutics that influence disease prognosis and survival rates11-13.
The traditional belief that agricultural populations live longer and are generally healthier compared to urban dwellers is constantly being challenged by epidemiological studies. Recent demographic data indicate that rural populations around the world are ageing at an accelerating pace14. This is increasing the overall burden of chronic disease, including cancer and diabetes15,16. Significant health disparities have been recorded for these types of pathologies and well-described socioeconomic variables have been proposed as modifiable risk factors17.
Epidemiological evidence on cancer mortality for underprivileged populations is important because it can be used to increase health awareness and help orchestrate interventions that mitigate disease impact. There is no doubt that early diagnosis through effective screening can significantly improve survival17. Prostate, breast and colorectal adenocarcinomas can be readily detected by digital rectal exam (DRE) and/or prostate-specific antigen (PSA) screening, mammography and colonoscopy, respectively, and this can have a direct impact on disease prevention18-21.
Within this spectrum of understanding, recording differential patterns of cancer mortality is mandatory for setting priorities in social, temporal and geographic contexts. Small-area epidemiological observations can unmask differences that are otherwise lost in the 'big picture' of national statistics22. This changes the agenda from whether it is suitable to use rural-urban disparities in the study of cancer to finding the best way of exploiting the information that emerges from comparisons of this kind23.
In the present study, two regions of north-eastern Greece that differ markedly were examined, one being predominantly agricultural and the other highly urbanized. Age-standardized mortality ratios were used to address corresponding differences between the two regions and conclusive evidence for the impact of various types of cancer have been devised. Based on these findings, some explanations for the observed discrepancies are suggested and public health interventions associated with the mitigation of the burden of cancer are proposed.
Mortality data for the period 1999-2008 were collected from the death registries of two regions: region A (N=3879 records), which was 92.7% urbanized (National Statistical Authority, 2001 census) and region B (N=2237 records), where 14 out of 15 communities were classified as rural. A population is defined as rural when fewer than 2000 residents are reported in the census. Cases involved male and female individuals with permanent residence in either of the two regions. Cause of death was defined according to the WHO's 10th edition of International Classification of Disease. Age-standardization was performed by the direct method using the WHO Standard European Population24 as reference, according to the equation:
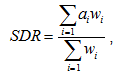
where 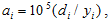 di are the number of deaths per age group, yi the corresponding person-years and wi the number of people in each age group (i) of the standard population.
di are the number of deaths per age group, yi the corresponding person-years and wi the number of people in each age group (i) of the standard population.
Calculation of the standard error (SE) was performed according to the equation:
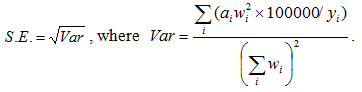
Confidence intervals at the 95% level (95%CI) were calculated by the Poisson approximation method:
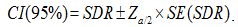
Finally, for direct comparisons rate ratios were calculated by the formula:
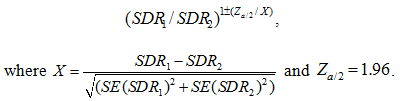
Standardized mortality rates for stomach (C16), colorectal (C18-20), pancreatic (C25), liver (C22), bladder (C67), kidney (C64), lung (C34), brain (C71) and lymphatic/hematopoietic (C81-96) cancers, as well as for total cancers of the digestive system (C15-26), including or excluding liver cancer, were analyzed in both sexes. Liver neoplasms were excluded from digestive system cancers, due to the possibility of secondary localization (metastasis) and other risk factors such as viral hepatitis and liver cirrhosis, which may artificially increase their overall impact. Moreover, standardized rates for prostate cancer (C61) were computed for males, and breast (C50), endometrial (C54) and ovary (C56) cancer for females.
All calculations were conducted in Microsoft Excel and the Statistical Package for the Social Sciences v15 (IBM; http://www.spss.com).
Ethics approval
This study has been approved by the Municipality of Evros Ethical Committee (Prot. No. 1441, 24-11-2008) and fully conforms to the provisions of the Declaration of Helsinki.
Crude and standardized, all cause-mortality comparisons for the two regions are given in Table 1. There was no overall effect of rurality/urbanicity on all cause mortality (RR=1.05, 95%CI=0.89-1.14). Moreover, there were no statistically significant differences between the two regions as regards circulatory diseases (I00-I99), respiratory diseases (J00-J99), infectious diseases (A00-B99) and external causes and injuries (V01-Y98).
Table 2 shows the age-standardized mortality per 100 000 person-years for various types of cancer in region A and region B. Comparisons are illustrated by rate ratios and associated 95%CI. Statistically significant differences were observed for cancers of the digestive system, excluding liver neoplasms (RR=1.02, 95%CI=1.02-1.54). Liver neoplasms were excluded from digestive system cancers, due to the possibility of secondary localization (metastasis) and other risk factors such as viral hepatitis and liver cirrhosis, which may artificially increase their overall impact. Mortality from stomach, colorectal, bladder and lymphatic/hematopoietic cancers, as well as liver, kidney and lung cancer, did not differ statistically between the two regions.
Prostate cancer mortality was positively and strongly associated with rurality (RR=1.86, 95%CI=1.10-3.14). Conversely, no significant differences were recorded for breast, ovarian and endometrial cancer among rural and urban females. Moreover, there were no other sex-specific disparities in cancer mortality for any other type of malignancy (Table 3).
Detailed analysis of age-specific cancer mortality produced some notable results (Fig1). The incidence of stomach cancer in rural areas was significantly elevated in the older age groups (>65 years). In contrast, there were no liver and lung cancer cases in younger cohorts of region A.
Table 1: General all-cause mortality per 100 000 person years (both sexes)

Table 2: Rural-urban disparities in cancer mortality: age-standardized rates and ratios
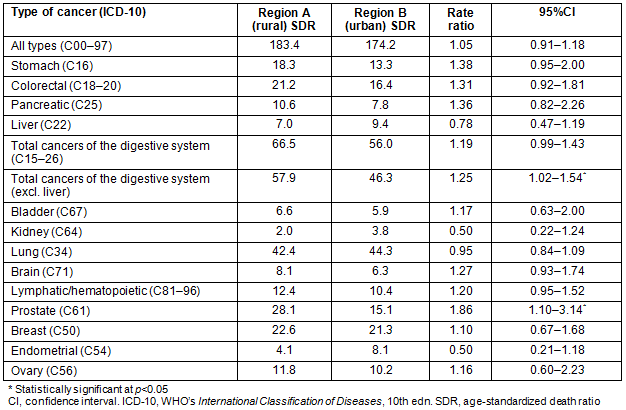
Table 3: Sex-specific age-standardized cancer mortality
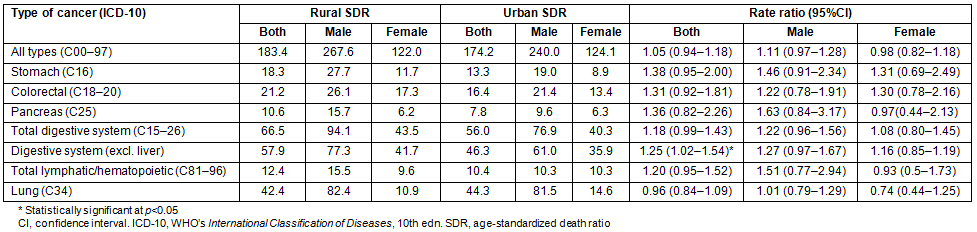
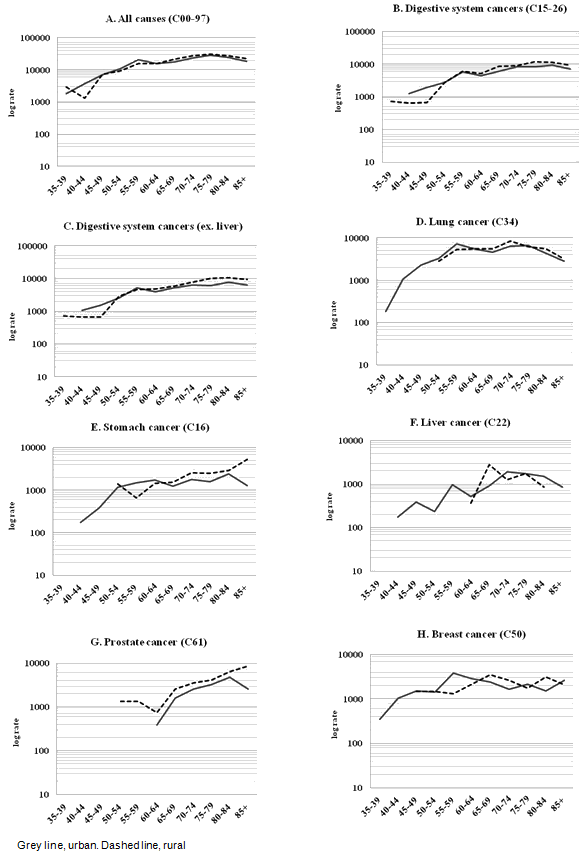
Figure 1: Age-standardized cancer mortality (semi-logarithmic scale).
Main findings of this study
This study has generated some interesting insight into cancer mortality with respect to rural-urban geographic reference in north-eastern Greece. Notably, the burden for prostate cancer in rural males was significantly higher when compared to their urban counterparts. Moreover, the incidence of stomach cancer in rural areas was significantly elevated in the older age groups (>65 years), indicating a historical discrepancy between the two regions. In contrast, there was a complete absence of liver and lung cancer cases in younger cohorts from rural areas, which can be attributed to structural differences in the population.
Discussion in light of the literature
Health disparities have been observed between rural and urban regions around the world. Several risk factors have been described as potential drivers of this epidemiological polarization. Access to health care, including distance from medical facilities, physician-to-population ratio, availability of cancer detection technologies and screening methods, constitute some of the most important aspects associated with social deprivation and rurality25-27. Limited financial resources and economic factors tend to augment these disparities even further. Moreover, the availability of public versus private medical centers and public health insurance coverage costs appears to be critical factors28.
Health promotion and education are often trivial in rural populations. Absence of disease control and prevention can lead to increased incidence and mortality. Behavioral factors such as smoking, diet and alcohol consumption may alter individual outcomes, although cultural or religious beliefs may be equally important29. Higher levels of stoicism and fatalism have been observed among rural populations, arising from the denial of presenting symptoms and the fear of stigmatization30. This may result in increased time to diagnosis, which ultimately leads to heavier tumor burden and worse treatment compliance.
This can be best exemplified in the case of prostate cancer. Prostate cancer typically develops slowly and the tumor may be preceded by dysplastic lesions for many years or even decades. Small and localized prostate neoplasia can thus remain unrecognized for many years before progressing to a clinically significant state. In many autopsies prostate cancers are found incidentally, suggesting that prostate hyperplasia is an inevitable feature of male physiology that comes with advanced age19. On the other hand, the introduction of PSA testing to detect prostate cancer has shifted the spectrum of diagnosed cancers from undifferentiated to moderately differentiated tumors (Gleason sum scores 5-7)31. Moreover, PSA screening has altered the age distribution of prostate cancer cases in many developed countries. In Germany, the mean age at diagnosis has declined from 73 years in 1980 to 69 years in 200632. The efficacy of the test has raised serious concerns, leading to suggestions for more selective use in high-risk groups only33. Nevertheless, early diagnosis of prostate cancer remains the best option as for all cancer types. In fact, 5-year prostate cancer survival rates are close to 100% for local or regional tumors. Conversely, when distant (stage IV, M1) tumors are considered, 5-year survival rates drop to less than 30%21.
Implications of this study
In this study, the comparison between rural and urban regions has indicated a clear discrepancy regarding prostate cancer mortality. In rural residents, mortality from this type of malignancy was 1.86 times higher when compared to their urban counterparts. This tendency was observed across all age groups, indicating an effect that is independent of age. Moreover, it suggests that the potential modifying factors are probably resilient to population characteristics and thus not crudely determined. A possible explanation for this phenomenon could be the socioeconomic status of agricultural populations. Lack of health attitudes towards preventive (early) screening and diagnosis, in combination with limited medical resources, including the absence of a specialist urologist/andrologist and diagnostic facilities in the area, may predispose for higher prostate cancer morbidity and mortality33.
A different picture emerges for cancers of the digestive tract. These are principally associated with stomach, colorectal cancers and pancreatic neoplasia (>85% of cases). Only marginal differences were observed between rural and urban areas. For stomach cancer, a probable cohort effect has been observed. In rural areas, the mortality from this type of malignancy was higher in the older age groups. This finding addresses a cultural aspect of cancer epidemiology. Global trends of stomach cancer have been declining since the 1950s34. This is due to the dietary transition brought about by the advances in food preservation, the introduction of refrigerators and the overall improvement in the quality of nutrition. This development has led to a decrease in the consumption of salt-preserved food and cured meat, such as pickled or smoked products, and an increase in the availability of fresh substitutes35. Technological and dietary advances have thus caused a gradual decline in stomach cancer incidence and mortality on a global scale36. However, the cohort of individuals that are over 65 years of age were born between 1934 and 1943 or earlier. This suggests that they may have been exposed to these risk factors for a longer period compared to their urban counterparts, and this may have contributed to their overall burden in developing this type of malignancy.
Impact of the study: strengths and limitations
A clear distinction is drawn between rural and urban regions as regards cancer mortality in north-eastern Greece. Although this study focuses only in two areas representing a small portion of the total population, it may well serve as a general paradigm. Further research is required to establish a full picture of the epidemiological map and assess temporal trends, to confirm this hypothesis on a wider scale. This will then enable intervention studies to be conducted to mitigate the effects of morbidity and mortality. Health promotion and education towards preventive measures, including the benefits of early screening, must be pursued, along with improved access to medical and diagnostic centers37,38. Reversing socioeconomic inequalities is the ultimate goal for supporting the health status of rural populations and this goal should be in line with central government policies aiming at more equal distribution of funds and resources. A constant monitoring of social, environmental and epidemiological parameters is necessary, in order to meet public health indicators and sustain long-term quality of life.
For medical practitioners in the rural sector this poses a true challenge. It is mandatory that appropriate training is provided, given that rural populations face special problems, such as ageing-related morbidity and polypharmacy. Creating the appropriate health culture at the primary (prevention) and secondary (screening) level would be beneficial both for the healthcare system and the local communities. This is best envisaged in programs that take into account performance and deliverance as integral components of the developmental process and economic planning.
Acknowledgements
The authors are grateful to Dr Anna-Betina Haidich (Aristotle University of Thessaloniki, Greece) and Mr Vasilis Nikolaou (University of Exeter, UK) for helpful discussions during the preparation of this manuscript.
References
1. Monroe AC, Ricketts TC, Savitz LA. Cancer in rural versus urban populations: a review. Journal of Rural Health 1992; 8(3): 212-220.
2. Swaminathan R, Selvakumaran R, Esmy PO, Sampath P, Ferlay J, Jissa V, et al. Cancer pattern and survival in a rural district in South India. Cancer Epidemiology 2009; 33(5): 325-331.
3. Omran AR. The epidemiologic transition. A theory of the epidemiology of population change. 1971. Bulletin of the World Health Organization 2001; 79(2): 161-170.
4. Hendryx M, Fedorko E, Halverson J. Pollution sources and mortality rates across rural-urban areas in the United States. Journal of Rural Health 2010; 26(4): 383-391.
5. Kinlen LJ, Petridou E. Childhood leukemia and rural population movements: Greece, Italy, and other countries. Cancer Causes & Control 1995; 6(5): 445-450.
6. Parikh-Patel A, Bates JH, Campleman S. Colorectal cancer stage at diagnosis by socioeconomic and urban/rural status in California, 1988-2000. Cancer 2006; 107(5 Suppl): 1189-1195.
7. Dey S, Zhang Z, Hablas A, Seifeldein IA, Ramadan M, El-Hamzawy H, et al. Geographic patterns of cancer in the population-based registry of Egypt: possible links to environmental exposures. Cancer Epidemiology 2011; 35(3): 254-264.
8. Goodridge D, Lawson J, Rennie D, Marciniuk D. Rural/urban differences in health care utilization and place of death for persons with respiratory illness in the last year of life. Rural and Remote Health 2010; 10(2): 1349.
9. Celaya MO, Berke EM, Onega TL, Gui J, Riddle BL, Cherala SS, et al. Breast cancer stage at diagnosis and geographic access to mammography screening (New Hampshire, 1998-2004). Rural and Remote Health 10(2): 1361. (Online) 2010. Available: www.rrh.org.au (Accessed 24 November 2013).
10. Rucker D, Hemmelgarn BR, Lin M, Manns BJ, Klarenbach SW, Ayyalasomayajula B, et al. Quality of care and mortality are worse in chronic kidney disease patients living in remote areas. Kidney International 2011; 79(2): 210-217.
11. Vanderpool RC, Kornfeld J, Mills L, Byrne MM. Rural-urban differences in discussions of cancer treatment clinical trials. Patient Education and Counseling 2011; 85(2): e69-e74.
12. Benuzillo JG, Jacobs ET, Hoffman RM, Heigh RI, Lance P, Martinez ME. Rural-urban differences in colorectal cancer screening capacity in Arizona. Journal of Community Health 2009; 34(6): 523-528.
13. Onega T, Duell EJ, Shi X, Wang D, Demidenko E, Goodman D. Geographic access to cancer care in the U.S. Cancer 2008; 112(4): 909-918.
14. Andrews GR. Demographic and health issues in rural aging: a global perspective. Journal of Rural Health 2001; 17(4): 323-327.
15. Melidonis AM, Tournis SM, Kompoti MK, Lentzas IL, Roussou VR, Iraklianou SL, et al. Increased prevalence of diabetes mellitus in a rural Greek population. Rural and Remote Health 6(1): 534. (Online) 2006. Available: www.rrh.org.au (Accessed 24 November 2013).
16. Lin JD, Zhang L, Xu ZZ, Xu LC. Research on burden of chronic diseases among rural-urban residents in Xuzhou. Public Health 2010; 124(6): 345-349.
17. Papastergiou P, Rachiotis G, Polyzou K, Zilidis C, Hadjichristodoulou C. Regional differences in mortality in Greece (1984-2004): the case of Thrace. BMC Public Health 2008; 23(8): 297.
18. Yu XQ. Socioeconomic disparities in breast cancer survival: relation to stage at diagnosis, treatment and race. BMC Cancer 2009; 9: 364.
19. Dunn MW, Kazer MW. Prostate cancer overview. Seminars in Oncology Nursing 2011; 27(4): 241-250.
20. Ugarte MD, Etxeberria J, Goicoa T, Ardanaz E. Gender-specific spatio-temporal patterns of colorectal cancer incidence in Navarre, Spain (1990-2005). Cancer Epidemiology 2012; 36(3): 254-262.
21. Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiology, Biomarkers & Prevention 2010; 19(8): 1893-1907.
22. German RR, Fink AK, Heron M, Stewart SL, Johnson CJ, Finch JL, et al. The accuracy of cancer mortality statistics based on death certificates in the United States. Cancer Epidemiology 2011; 35(2): 126-131.
23. Singh GK, Siahpush M, Williams SD. Changing urbanization patterns in US lung cancer mortality, 1950-2007. Journal of Community Health 2012; 37(2): 412-420.
24. Ahmad OB, Boschi-Pinto C, Lopez AD, Murray CJL, Lozano R, Inoue M. Age standardization of rates: a new WHO standard. GPE discussion paper series: no. 31. Geneva: World Health Organization, 2001.
25. Gartner A, Farewell D, Roach P, Dunstan F. Rural/urban mortality differences in England and Wales and the effect of deprivation adjustment. Social Science & Medicine 2011; 72(10): 1685-1694.
26. Stamenic V, Strnad M. Urban-rural differences in a population-based breast cancer screening program in Croatia. Croatian Medical Journal 2011; 52(1): 76-86.
27. Doescher MP, Jackson JE. Trends in cervical and breast cancer screening practices among women in rural and urban areas of the United States. Journal of Public Health Management and Practice 2009; 15(3): 200-209.
28. Perez-Gomez B, Aragones N, Gustavsson P, Lope V, Lopez-Abente G, Pollan M. Socio-economic class, rurality and risk of cutaneous melanoma by site and gender in Sweden. BMC Public Health 2008; 8: 33.
29. Singh GK, Hoyert DL. Social epidemiology of chronic liver disease and cirrhosis mortality in the United States, 1935-1997: trends and differentials by ethnicity, socioeconomic status, and alcohol consumption. Human Biology 2000; 72(5): 801-820.
30. Howat A, Veitch C, Cairns W. A descriptive study comparing health attitudes of urban and rural oncology patients. Rural and Remote Health 6(4): 563. (Online) 2006. Available: www.rrh.org.au (Accessed 24 November 2013).
31. Iczkowski KA, Lucia MS. Current perspectives on Gleason grading of prostate cancer. Current Urology Reports 2011; 12(3): 216-222.
32. Robert-Koch-Institut. Krebs in Deutchland 2003-2004. Berlin: Robert-Koch-Institut Publications, 2008.
33. Moyer VA. U.S. Preventive Services Task Force. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Annals of Internal Medicine 2012; 157(2): 120-134.
34. Shibata A, Parsonnet J. Stomach cancer. 3rd ed. New York: Oxford University Press, 2006.
35. Plummer M, Franceschi S, Munoz N. Epidemiology of gastric cancer. IARC Scientific Publications 2004; (157): 311-326.
36. Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA: A Cancer Journal for Clinicians 2008; 58(2): 71-96.
37. Swaminathan R, Selvakumaran R, Vinodha J, Ferlay J, Sauvaget C, Esmy PO, et al. Education and cancer incidence in a rural population in south India. Cancer Epidemiology 2009; 33(2): 89-93.
38. Sabesan S, Piliouras P. Disparity in cancer survival between urban and rural patients - how can clinicians help reduce it? Rural and Remote Health 9(3): 1146. (Online) 2009. Available: www.rrh.org.au (Accessed 24 November 2013).
