Introduction
In 1996, the Federal, State and Territory Governments of Australia identified diabetes mellitus as one of six National Health Priority Areas. The Australian Diabetes, Obesity and Lifestyle Study, which arose from the National Diabetes Strategy and Implementation Plan1, determined that approximately 940 000 Australians over the age of 25 years had diabetes2. Furthermore, the prevalence of diabetes in Australian adults had trebled since 1981 and for every known case of diabetes there was one undiagnosed case2.
Recently, in the Australian state of Victoria, diabetes was recognised as one of the top 10 health problems for people of all ages in the rural 'Mallee Track' region (centred on the country town of Ouyen, approximately 400 km north-west of Melbourne, the capital of Victoria [Fig. 1])3. In an attempt to improve diabetes services in this region, the Mallee Track Health & Community Service (MTH&CS), based in Ouyen, undertook a project entitled 'Diabetes Management Along the Mallee Track'. This project was funded by a Rural Chronic Disease Initiative (RCDI) program grant from the Australian Government's Department Health and Ageing, Canberra, and was based in part on the MAN Model of Health Promotion, piloted and developed by Centre for Advancement of Men's Health across rural Victoria4.
Figure 1: Location of Ouyen, the central town in the Mallee Track region of Victoria.
The primary aims of the Diabetes Management Along the Mallee Track project were:
- To identify people at risk for diabetes and raise the level of awareness about diabetes in the general community, through the delivery of community-based risk assessment programs across the region.
- To provide improved services for people with established diabetes across the region, through the establishment of an integrated, multidisciplinary, 'one-stop' service for the management of diabetes.
The novel use of point-of-care (POC) pathology testing was a key component of both the risk assessment and management arms of the project. POC testing (POCT) is a major growth area within community and hospital medicine in Australia, and is soon to be trialled within the general practice sector in Australia5. POCT provides the opportunity for pathology tests to be performed on-site in the community by a trained health professional, with results available within 10-15 min.
This article describes the use of POCT for the risk assessment and management arms of the Mallee Track program (focussing particularly on the latter), and reports the level of satisfaction among community members with diabetes, their doctors and allied health professionals with the new POCT services provided as part of this project. An initial assessment on clinical outcome measures for patients with diabetes, one-year post-introduction of the program, is made.
Methods
Description of the Mallee Track Region
The Mallee Track region of north-west Victoria is a classified as a remote area, according to the Australian Government's rural and remote areas (RRMA) classification system6. It is more than 350 km from the nearest capital cities (Adelaide and Melbourne) and 100 km from the nearest rural centre, Mildura. Agriculture (wheat and barley) and sheep farming are the region's main local industries. There are three main towns in the region, Ouyen, Underbool and Murrayville, Ouyen having the largest population of approximately 1150 people (690 adults). The region's total population is approximately 2800 (1680 adults).
Description of diabetes services prior to the introduction of the program
Prior to the introduction of the program, diabetes services for patients were disjointed and uncoordinated. Local services provided by two GPs were used sporadically by only 15-20 patients with diabetes. Patients had to travel considerable distances (100 km) to obtain selected specialist services (such as diabetes education) and had to wait several days for pathology results. On average each patient had one haemoglobin A1c (HbA1c) measurement performed annually to assess his or her diabetes control.
Partnerships
The Diabetes Management Along the Mallee Track project was established and directed by the Special Community Health Projects Team from MTH&CS, in partnership with local GPs and MTH&CS Allied Health and community health nurses. A diabetes educator visited the Mallee Track Medical Centre monthly through a partnership with the Mallee Division of General Practice. A podiatrist was also engaged locally. The Community Point-of-Care Services unit from the Flinders University Rural Clinical School supported the project with POC technology, training and competency certification for local POCT operators, quality management procedures, data management and assistance in designing community surveys. A local Advisory Committee was formed to provide direction and guidance to the program; the committee included local community representation from the Ouyen Diabetes Support Group.
Community-based risk assessment programs
Members of the Mallee Track community were invited to participate in community risk assessment sessions held in seven towns across the region: Ouyen, Murrayville, Walpeup, Underbool, Patchewollock, Speed and Manangatang. Participation in risk assessments was voluntary and opportunistic. Risk assessments were conducted in a local community 'settings' approach; for example, using the local Community Fire Authority (CFA), local schools and colleges, and community field days as venues for risk assessment sessions, as well as targeting specific community groups such as the local walking group and the local men's tennis club. Diabetes risk factor assessment was based on current Australian best practice guidelines7-11, and included random capillary blood glucose and total cholesterol (measured by fingerprick POCT), blood pressure, age, personal and family history of diabetes, smoking status, and self-assessed weight and level of exercise.
Management of people with established diabetes
Community members with established diabetes were invited to participate in a new multidisciplinary service, which involved a single appointment with their local GP. This appointment also included meeting the diabetes educator and podiatrist and on-site POC testing for HbA1c, urine albumin : creatinine ratio (ACR), blood lipids and glucose performed by the Special Community Health Projects team nurse. Having POCT results available within the single consultation enabled the GP to enact patient management and/or change treatment during the consultation, without the need for the patient to return for a follow-up visit. This integrated approach was designed to provide a more accessible and convenient service for people with diabetes, and to improve patient motivation to self-manage their diabetes. It also overcame the need for patients to travel long distances to access specialist services. A local register of all participants in this service was established, including POCT results conducted at the commencement of the program (0 months) and at every subsequent visit to their local GP. This enabled future tracking of diabetes control and an assessment of clinical outcomes post introduction of the new service.
POCT instruments
The Bayer DCA 2000 (Bayer Australia, Melbourne, Vic, Australia) and the Cholestech LDX Lipid Analyser (Point of Care Diagnostics, Sydney, NSW, Australia) were used for POCT. The Bayer DCA 2000 measured HbA1c on a fingerprick (1 mL) of whole blood in 6 min. HbA1c is a well-established biochemical marker that provides a measure of a person's diabetes control over the preceding 3 months1,2,7. The DCA 2000 is currently used for POC HbA1c monitoring for people with diabetes in 60 urban, rural and remote Australian Aboriginal medical services, through the 'QAAMS' program (Quality Assurance for Aboriginal Medical Services)12-14. The DCA 2000 has proven safe, analytically reliable and robust, and clinically and culturally effective in this setting12-15.
The DCA 2000 was also used to measure albumin:creatinine ratio (ACR) on 40 mL of urine in 7 min. Urine ACR is a key biochemical marker for the early detection of microalbuminuria and for monitoring the progression of diabetic nephropathy16. The instrument is used in the national "QAAMS" program for point-of-care ACR testing, its analytical performance has been validated against equivalent laboratory-based methods, and it is a useful test for the detection and management of chronic disease in a rural community setting14,17-19.
The Cholestech LDX machine measured total cholesterol, triglyceride, HDL-cholesterol, LDL-cholesterol (calculated) and glucose on a fingerprick (35 mL) of whole blood in 5 min. The analytical performance of the Cholestech machine has been validated in the laboratory and in the rural community setting20-21.
Quality management of the POCT equipment
The Flinders' Community Point-of-Care Services unit implemented an internal quality management program to monitor the analytical performance of the POC instruments in the field. Local operators of the POC equipment were required to test a commercially available quality control (QC) material for each POC test and on each POC instrument every time a new reagent kit was opened (containing 10 testing cartridges).
Questionnaires to assess project outcomes
With assistance from the Flinders University Centre for Epidemiology and Biostatistics, a questionnaire was developed to assess participants' views on the introduction of POC services and to assist policy development for the future planning and delivery of diabetes services along the Mallee Track region. The questionnaire design was based on the 5-point Likert scale22, with respondents recording their levels of agreement or disagreement with statements posed. Participants were given equal opportunity to agree or disagree with each statement. The questionnaire for people with established diabetes assessed their level of satisfaction with the POC testing services provided through the project, and was administered at the completion of the project (that is, after 12 months). The President of the Ouyen Diabetes Support Group assisted with the development of this questionnaire. The Manager of the MTH&CS Special Projects team explained the aims and objectives of the questionnaire to people with diabetes at a monthly meeting of the Ouyen Diabetes Support Group. Following this meeting each person filled out a questionnaire in his/her own time and at his/her convenience. Completed questionnaires were returned in a sealed envelope to the Manager of the MTH&CS Special Projects team within 2 weeks. Two further surveys were implemented. Three local GPs were surveyed to assess their satisfaction with the new POC testing services for diabetes management, and the three health professionals responsible for conducting POC testing also completed a questionnaire to gain information about their acceptance of the POC technology used during the project.
Statistical methods
The demographics and POC results of the participants who underwent community-based risk assessment were examined. Continuous, normally distributed variables were expressed as means and standard deviations (SD), and variables with skewed distributions were reported as medians and inter-quartile ranges (IQR). Categorical variables were reported as frequency and percentage. Comparisons were made between the POC measurements by gender, using Mann-Whitney U-tests. The prevalence of risk factors in community participants was reported, and their relationship with age was examined using c2 test for trend. For participants with established diabetes, group mean (SD) POC measurements were calculated at the program commencement and at their most recent visit to their local GP.
The results of the satisfaction questionnaires were reported as the number (and percentage) of respondents who agreed, were neutral, or disagreed with the statements presented in the questionnaires. Comparisons were made between the satisfaction with diabetes services provided prior to, and following the program implementation, using a c2 test.
Results
Risk assessment
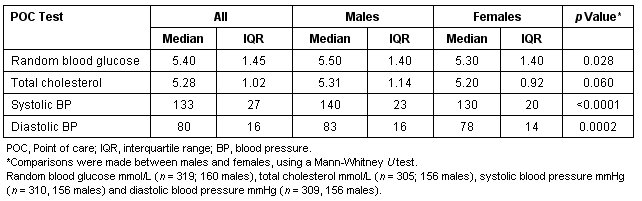
Three hundred and twenty people underwent risk assessment for diabetes during community sessions along the Mallee Track, over the study period. The mean age of those assessed was 50.3 years (SD 14.7, range 16-86 years). POC measurements collected at community risk assessment are described (Table 1). Male participants had higher random blood glucose and higher systolic and diastolic blood pressures, when compared with female participants (Mann-Whitney U-test, p<0.05).
Table 1: Baseline characteristics of POC measurements conducted during the community risk assessment program
Risk factors for diabetes among those assessed in the Mallee Track community were common, with over two-thirds (n = 210) having an equivocal capillary blood glucose (5.1-11.0 mmol/L) (Figure 2). 38% (n = 116) of those tested had abnormal lipids (total cholesterol ≥ 5.5 mmol/L), while 44% (n = 137) had hypertension (systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg). Smoking rates were low (13%, n = 37). These prevalence rates for people in the Mallee Track region are lower than the national average2 for lipids (38% vs 51% respectively) and smoking (13% vs 16%) but higher for hypertension (44% vs 29%). The latter finding is attributed to a very high rate of hypertension in males in the region (54% vs 31% nationally). A positive trend was identified between hypertension and increasing age (c2 = 24.5, p<0.001), and between abnormal lipids and increasing age (c2 = 12.8, p = 0.03). The prevalence of diabetes (random blood glucose ≥ 11.1 mmo/L) in those assessed was not associated with age (c2 = 11.2, p = 0.35), however the number of persons assessed in the lower age groups were small. Three new cases of diabetes were identified as a result of the risk assessments conducted.
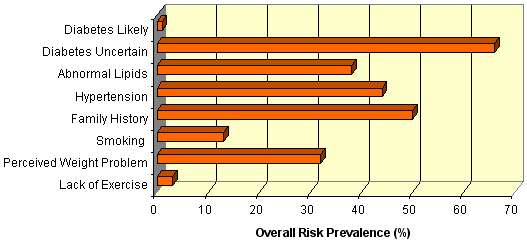
Figure 2: Prevalence of risk factors* for diabetes in the Mallee Track region. *Categorisation of key risk factors: diabetes unlikely, capillary blood glucose ≤ 5.0 mmol/L; diabetes uncertain, capillary blood glucose 5.1-11.0 mmol/L; diabetes likely, capillary blood glucose ≥ 11.0 mmol/L; abnormal lipids, total cholesterol ≥ 5.5 mmol/L; hypertension, sBP ≥ 140 mmHg or dBP ≥ 90 mmHg.
Management of established diabetes
Forty-nine persons with established diabetes commenced POC pathology testing at their local general practice across the first 12 months of the program, with another five people with diabetes joining this group over the following 6 months. A local diabetes register was established and all POCT results were entered into this register following each GP visit. The MTH&CS and the Flinders' Community Point-of-Care Services unit jointly maintain the register, which is electronically updated and available to local general practitioners.
These diabetes patients (n = 54) have now been monitored by POCT for a mean of 10 months (range 3-18 months). A total of 162 POCT HbA1c tests, 91 ACR tests, and 132 lipids tests have been performed since POCT commenced. The percentage of diabetes patients achieving optimal glycaemic control (HbA1c <7%) increased from 33% (start of the program) to 63% (POCT measurement at their most recent visit to the GP). The percentage achieving controlled glycaemia (HbA1c < 8%) increased from 59% to 91%, while the number exhibiting poor control fell from 13% to 6% (Figure 3). Since the commencement of POCT, the mean HbA1c, cholesterol, and systolic blood pressure of the diabetes group has fallen significantly (paired t-test) by 0.5%, 0.36 mmol/L and 9 mmHg as measured by POCT at their most recent visit to the GP (Table 2). Diastolic blood pressure had fallen by 5 mmHg.
Table 2: Mean (SD) of POCT measurements in the Diabetes Management group at the commencement of the program (0 months) and at their most recent visit to the GP (most recent)
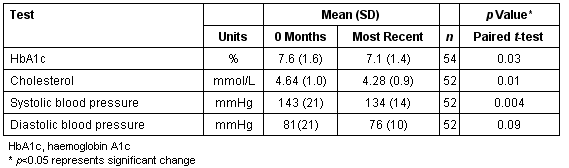
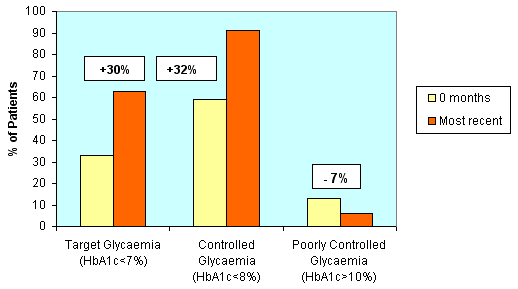
Figure 3: Improvement in glycaemic control among diabetes patients, showing an increase in percentage of diabetes patients attaining glycaemic targets and a decrease in the percentage of patients exhibiting poor control, as measured by POCT at the commencement of the program (0 months) and at their most recent visit to the GP.
Quality management of POC instruments
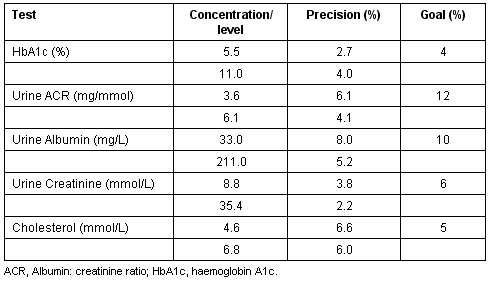
Twenty-two Bayer HbA1c QC tests, 17 Bayer Urine ACR QC tests and 44 Cholestech lipid QC tests were conducted during the project period (Table 3). The precision of internal quality control testing for HbA1c, urine ACR, urine albumin and urine creatinine met the desirable analytical performance specifications recommended by the Australian Government's 'Interim Standards for Point of Care Testing in General Practice'23. For total cholesterol, the precision achieved for quality control testing was very close to the recommended analytical goal.
Table 3: Precision achieved for internal quality control testing on POC instruments
Satisfaction with New Management Services for Diabetes
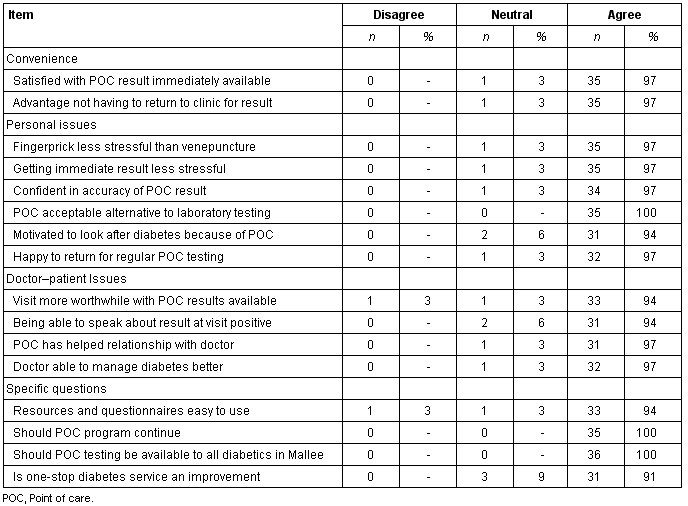
Satisfaction questionnaires were received from 36 people with established diabetes, two-thirds (n = 28) of whom were aged 55 years or older. This number of respondents represented 73% (36/49) of all persons in the Mallee Track region known to have diabetes at the time the questionnaires were implemented. The majority of respondents (> 90%) reported a high level of satisfaction with the convenience of the program, personal issues, doctor-patient issues and the program overall (Table 4). There was unanimous agreement among respondents that they would like to see POC testing continue for their own diabetes management, and that they wished POC testing to be available to all people with diabetes across the Mallee Track region.
Table 4: Results of questionnaire on POC testing for people with established diabetes
The proportion of respondents who were satisfied/very satisfied with the available diabetes services was significantly greater following the introduction of the project (before: n = 18 [64%], after: n = 29 [91%], c2 = 6.10, p = 0.01) (Figure 4).
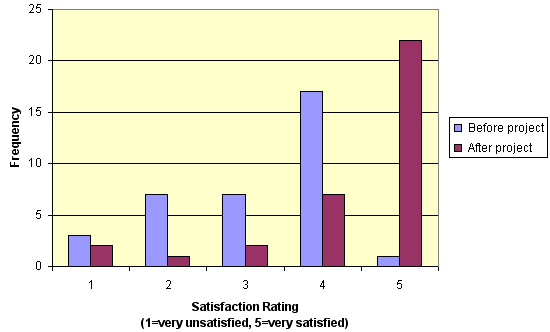
Figure 4: Comparison of the overall level of satisfaction with diabetes services before and after the Diabetes Management Along the Mallee Track project.
Three local GPs completed a satisfaction questionnaire. All agreed that the availability of POCT during consultations was convenient and the opportunity to discuss POC results immediately was advantageous. They had confidence in the accuracy and reliability of the POC result and believed it was an acceptable alternative to laboratory testing. They also agreed that immediate availability of the POC result contributed positively to overall patient care and patient compliance, as well as improving their rapport and relationship with the client. All three strongly agreed that the POCT component of the program made a positive contribution to the management of diabetes within their service.
Three community health nurses responsible for conducting POCT completed a satisfaction questionnaire. All agreed that the education, training and resources provided by the Flinders' Community Point-of-Care Services unit were useful and appropriate. They had confidence in the accuracy and reliability of the POC results and understood the need to perform quality control testing. Among the three respondents, there was general agreement that patients in the community were happy with POCT services, the program had provided a focus for raising community awareness about diabetes and enhanced the sense of community ownership of the project.
Discussion
The emergence of POC pathology testing throughout the world has paralleled significant advances in medical technology, changes in healthcare delivery, with a more patient-orientated approach to care and an increasing demand for improved turnaround of pathology results24. The Australian Federal Government recently commissioned a major review of the role and value of POCT in the general practice environment25. This review concluded that only very limited information is currently available on the efficacy of POCT in general practice in Australia26 but that rural and remote practices could potentially be the greatest beneficiaries of POCT.
In the rural-based Diabetes Management Along the Mallee Track Project, POCT was introduced for both risk assessment and the management of diabetes. The use of the DCA 2000 and Cholestech LDX POC technology for this purpose has proven safe, robust and analytically reliable in rural community hands. As part of the risk assessment sessions, POCT contributed to a greater community understanding of diabetes and its associated risk factors and provided an effective and rapid means for on-going surveillance of community risk. The coordinated, multidisciplinary 'one-stop' approach to diabetes management, combining access to GP and specialist support services with on-site POCT and immediate result availability, has been well supported by the region's diabetes patients. There are now more than two-and-a-half times more patients accessing this new service and receiving closer monitoring of their diabetes control compared to the number utilising the previous diabetes service. The number of patients achieving glycaemic targets has increased greatly, while improvements in diabetes, lipid and blood pressure control have also occurred. Patients with diabetes have expressed a significantly higher level of satisfaction with the new diabetes service, although there is potential for retrospective bias. They were unanimous that they wanted POCT to continue for their personal diabetes management and that POCT should be available for all people with diabetes in the region. Local doctors and health professionals conducting POCT were confident with this mode of health service delivery.
Two key challenges for the program are: (i) the on-going maintenance of the local diabetes register and the commitment to continue performing key POC pathology tests at the frequency recommended for best practice management; and (ii) attention to the care and follow up of people identified at greatest risk for diabetes via the community risk assessment sessions.
The Diabetes Management Along the Mallee Track project was initially selected as one of 19 innovative rural projects funded through the Australian Government's RCDI program. At the conclusion of this RCDI program, the Government selected the Diabetes Management Along the Mallee Track project as one of three demonstration projects for showcasing to all rural and remote health services in Australia through the production of an education resource called Building Healthy Communities27. This resource features a DVD and video on how the Diabetes Management Along the Mallee Track project is conducted on a day-to-day basis and aims to provide a framework to assist rural communities throughout Australia to conduct more effective community projects.
While acknowledging the population sample size in this project was relatively small and a larger study would be needed to broaden the generalisability of our findings, the Mallee Track model, with its associated POCT services, has considerable potential to be tailored locally and applied to many similar rural and remote health services in Australia, where community will and health professional commitment can work together for the common cause of reducing the prevalence and burden of diabetes.
Acknowledgements
The Diabetes Management along the Mallee Track project was supported by a Rural Chronic Disease Initiative program grant from the Australian Government's Department of Health and Ageing, Canberra (2003). Servier Laboratories (Australia) generously sponsor the Community Point-of-Care Services unit at Flinders University. Dean Whiting from Bayer Australia (Melbourne), Rupert Haines from Point of Care Diagnostics Australia Pty Ltd (Sydney) and Pfizer Australia kindly provided equipment, reagents and consumables for the project. Mr John Senior (CEO of the Mallee Track Health & Community Service) is thanked for his encouragement and support during the program.
References
1. Colagiuri S, Colagiuri R, Ward J. National Diabetes Strategy and Implementation Plan. Canberra: Diabetes Australia, 1998.
2. Dunstan D, Zimmet P, Wellborn T et al. Diabesity and associated disorders in Australia 2000. The accelerating epidemic. Melbourne: International Diabetes Institute, 2001.
3. Victorian Burden of Disease Study. Public Health and Development Division. Melbourne, Vic: Department of Human Services, July 1999.
4. Denner B. MAN model: Health Promotion. Australian Journal of Primary Health Interchange 2000; 6: 231-240.
5. Australian Government Department of Health and Ageing. Design for a trial and an evaluation framework for the introduction of point of care testing into General Practice in Australia. (Online) 2003. Available: http://www.health.gov.au/pathology/pdf/design.pdf (Accessed 23 May 2005).
6. Rural, Remote and Metropolitan Areas Classification. Department of Primary Industries and Energy and Department of Human Services and Health. Canberra, ACT: Australian Government Publishing Service, 1994.
7. NHMRC. NHMRC National Evidence based Guidelines for the Management of Type 2 Diabetes Mellitus. (Online) 2001. Available: http://www.nhmrc.gov.au/publications/synopses/cp86syn.htm (Accessed 11 July 2005).
8. Diabetes Australia and the Royal Australian College of General Practitioners. Diabetes Management in General Practice 2004/5. Diabetes Australia Publication NP 1055. Tuggerah, NSW: Diabetes Australia, August 2004.
9. Australian Diabetes Society. Diabetic dyslipidaemia - Australian Diabetes Society position statement. Medical Journal of Australia 1995; 162: 91-93.
10. National Heart Foundation of Australia and Cardiac Society of Australia and New Zealand. Lipid Management Guidelines - 2001. Medical Journal of Australia 2001; 175: S57-S85. Note page 78.
11. Guidelines Subcommittee. 1999 World Health Organisation - International Society of Hypertension Guidelines for the management of hypertension. Journal of Hypertension 1999; 17: 151-183.
12. Shephard MDS, Gill J. Results of an Innovative Education, Training and Quality Assurance Program for Point-of-Care HbA1c Testing using the Bayer DCA 2000 in Australian Aboriginal Community Controlled Health Services. Clinical Biochemist Reviews 2003; 24: 123-131.
13. Shephard MDS, Mundraby K. Assisting diabetes management through point-of-care HbA1c testing - "QAAMS" program for Aboriginal health workers. Aboriginal and Islander Health Worker Journal 2003; 27(4): 12-18.
14. Shephard MDS. Point-of-care testing in the Indigenous Rural Environment - the Australasian Experience. In: C Price, J Hicks, A St John (Eds). Point-of-Care Testing. Washington DC: AACC Press, 2004; 293-301.
15. Brice G, Daley L, Bellis-Smith N. From 'Major Threat' to 'Major Opportunity'? Pilot Project to Assess the Use of On-site Haemoglobin A1c Testing for Managing Persons with Diabetes (using the Bayer DCA 2000 analyser) in Australian Aboriginal Community Controlled Health Services: June 1999 to August 2000. Deakin West, ACT: National Aboriginal Community Controlled Health Organisation, 2001.
16. Sacks DB, Bruns DE, Goldstein DE, Maclaren NK, McDonald JM, Parrott M: Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clinical Chemistry 2002; 48: 436-472.
17. Shephard MDS, Allen GG, Barratt LJ et al. Albuminuria in a remote South Australian Aboriginal community: results of a community-based screening program for renal disease. Rural and Remote Health 3: 156. (Online) 2003. Available http://rrh.org.au (Accessed 23 May 2005).
18. Shephard MDS, Allen G. Screening for renal disease in a remote Aboriginal community using the Bayer DCA 2000. Australian Journal of Medical Science 2001; 22: 164-170.
19. Shephard MDS, Gill JP. An innovative Australian point-of-care model for urine albumin : creatinine ratio testing that supports diabetes management in indigenous medical services and has international application. Annals of Clinical Biochemistry 2005; 42: 208-215
20. Shephard M, Tallis G. Assessment of the point-of-care Cholestech lipid analyser for lipid screening in Aboriginal communities. Australian Journal of Medical Science 2002; 23: 4-10.
21. Jones R, Mazzachi B, Shephard M. Point-of-Care in Aboriginal Hands. Aboriginal and Islander Health Worker Journal 2002; 26: 13-16.
22. Estermann A. The Likert scale. Australian Epidemiologist 2003; 10: 46-48.
23. Shephard MDS. Analytical performance criteria for point-of-care testing instruments. In: Standards for Point of Care Testing in General Practice. Incorporating POCT Trial Guidelines. Canberra, ACT: Australian Government Department of Health and Ageing, 2004; 101-140.
24. Price CP, St John A, Hicks J. Point-of-care testing: what, why, when and where? In: C Price, J Hicks, A St John (Eds). Point-of-Care Testing. Washington DC: AACC Press, 2004: 3-9.
25. Guilbert R, Schattner P, Sikaris K, Churilov L, Leibowitz R, Matthews M. Review of the role and value of near patient testing in general practice. Report to the Pathology Section, Diagnostic and Technology Branch, Commonwealth Department of Health and Aged Care. (Online) 2001. Available: http://www.health.gov.au/internet/wcms/publishing.nsf/content/health-pathology-poctt-index.htm (Accessed 23 May 2005).
26. Cohen J, Piterman L, McCall L, Segal L. Near-patient testing for serum cholesterol: attitudes of general practitioners and patients, appropriateness, and costs. Medical Journal of Australia 1998; 168: 605-610.
27. Australian Government Department of Health and Ageing. Building Healthy Communities. A Guide for Community Projects. Canberra, ACT: Australian Government Department of Health and Ageing, 2004.






