Approximately 15% of US obstetric deliveries occur in rural settings1. Obstetrician-gynecologists (OBGYNs) provide the majority of obstetric care in the country; however, they disproportionately practice in urban settings, resulting in disparities in maternity care access for patients in rural areas2,3. In 2010, 49% of all US counties (home to 10.1 million women or 8.2% of all women) lacked an OBGYN2. Because of this, other obstetric providers, such as certified nurse-midwives (CNMs), family physicians, and general surgeons contribute substantially to the rural obstetric workforce4.
Access to health care in rural areas is further complicated by the fact that people living in rural communities are more likely than urban residents to have low incomes, lack health insurance, or rely substantially on Medicaid and Medicare2. Women in rural areas travel longer distances to access a range of medical services, including obstetric care; indeed, less than half of rural women live within a 30-minute drive of a hospital offering perinatal services5. Among pregnant women, rates of prenatal care initiation in the first trimester of pregnancy (a widely recommended practice) are lower for rural women, compared with those in urban and suburban areas6. Closure of obstetric units in rural hospitals has accelerated in recent years, further raising concerns about access for rural women7. Low patient volume and staffing difficulties are among the most frequently cited reasons for closures of rural obstetric units8. A recent Canadian study highlighted that rural maternity services are struggling to remain open and documented an association between travel distance for maternity services and adverse outcomes including perinatal mortality, which is highest in communities that are greater than 4 hours from maternity services9. Women from rural and remote communities with no local access to maternity services have worse maternal and newborn outcomes than women from similar communities with local access to even limited obstetric services10.
In addition to concerns related to obstetric care access for rural women, there are questions about the quality of obstetric care in rural areas11,12. One key metric of quality obstetric care is the use of early elective deliveries (EED). An EED is childbirth that occurs (via cesarean or labor induction) prior to 39 weeks completed gestation, in the absence of a medical indication. For more than 30 years, the American College of Obstetricians and Gynecologists (ACOG) and the Society of Maternal-Fetal Medicine have advocated delaying deliveries until 39 weeks of gestation or beyond in the absence of any medical indications13. At the same time, rates of cesarean delivery and labor inductions have increased dramatically in the USA, with labor induction rates more than doubling from 9.5% in 1990 to 22.1% in 200412. Although it is not clear what proportion of these inductions are elective, the overall rate of induction of labor is rising faster than the rate of pregnancy complications that would likely necessitate a medically indicated induction14-16.
Medical indications in pregnancy for which there is evidence to support delivery between 37 and 39 weeks include pre-eclampsia, gestational or chronic hypertension, oligohydramnios, placenta previa, multiple gestations, premature rupture of membranes, and fetal congenital malformations17. Medical conditions such as suspected macrosomia, well-controlled gestational diabetes, and documented pulmonary maturity do not require early-term delivery13. In these cases, early delivery may cause more harm than good; there are greater reported rates of morbidity and mortality among neonates and infants delivered during the early-term period compared with those delivered at terms of 39 and 40 weeks13,18. Higher rates of respiratory distress syndrome, transient tachypnea of the newborn, pneumonia, and surfactant and oscillator use were reported for infants delivered at 37 weeks of gestation compared with those delivered at 39 weeks of gestation13,19. Professional societies and hospitals across the USA have attempted to adopt policies to decrease the EED, and successful policies - notably, those that employ a 'hard stop', forbidding all elective deliveries before 39 weeks - have been shown to improve neonatal outcomes20-22.
Another aspect of the quality of obstetric care that is relevant for rural women is access to vaginal birth after cesarean (VBAC). The cesarean delivery rate in the USA has dramatically increased from 5% in 1970 to 23.5% in 1991 to 32.8% in 201123-25. The rise in cesarean delivery rates is attributable to both an increase in primary (first-time) cesareans and a decline in attempted trials of labor after cesarean (TOLAC)26. Of US women who require an initial cesarean delivery, more than 90% will have a subsequent cesarean26. In 1999, ACOG issued guidelines recommending that resources for emergency cesarean delivery (ie obstetric pediatric, anesthetic, and operating room staff) should be 'immediately available' for women undergoing TOLAC27. This recommendation presented a challenge in rural settings, where higher-acuity staff and resources are more limited28,29. In 2010, the National Institutes of Health (NIH) examined safety and outcomes of VBAC and factors associated with declining rates of VBAC30. The NIH recognized that attempting a VBAC was a reasonable option for many women with a prior cesarean delivery and called on clinicians and healthcare delivery systems to facilitate greater access to TOLAC30. More recently, Healthy People 2020 goals have focused on reducing by 10% the overall rate of repeat cesarean deliveries from 90.8% in 2007 to 81.7% by 202031.
Whether women in rural areas have access to obstetric care that is consistent with recent recommendations regarding EED and VBAC is not known. The purpose of this study was to describe EED and VBAC policies in rural maternity hospitals in the USA, and to measure whether these policies differed by staffing, facilities, or birth volume.
Data
A cross-sectional telephone survey of 306 rural maternity hospitals in the USA was conducted from November 2013 to March 2014. Detailed methods are published elsewhere4. Rural areas were defined based on the Office of Management and Budget non-metropolitan county definition. Briefly, a telephone survey of rural hospitals with obstetric units was conducted in nine states: Colorado, Iowa, Kentucky, New York, North Carolina, Oregon, Vermont, Washington, and Wisconsin. A total of 263 hospitals (86%) responded to the survey, with the majority of the survey respondents (95%) having a managerial role in the obstetrics or women's health department (eg director or obstetrical nurse manager)4. This survey was conducted by the University of Minnesota Rural Health Research Center and fielded by the Office of Measurement Services at the University of Minnesota4. A subset of these survey data was used to examine EED and VBAC among surveyed rural hospitals given that these are two obstetrical quality care markers for which policy can be implemented to influence practice, consistent with clinical evidence.
Measurement
Three questions within the survey specifically addressed EED. The first question was 'With regard to early elective delivery (induction or cesarean without medical indication between 37 and 39 weeks), has the hospital implemented either a hard stop policy (no early elective deliveries are allowed) or a soft stop policy (early elective deliveries only allowed in specific circumstances)?' The respondents could choose from 'yes, hard stop (no early elective deliveries are allowed)'; 'yes, soft stop (only allowed in specific circumstances)'; 'no, neither one'; and 'don't know'. The second question was 'Does the hospital track its early elective delivery rate?' The respondents could choose from 'yes', 'no' and 'don't know'. If respondents answered 'yes' to the second question, then, 'What is the early elective delivery rate for the current year?' was asked.
One question within the survey related to VBACs: 'Does the hospital allow vaginal birth after cesarean (VBAC) (eg trial of labor after previous cesarean)?' The respondents could then answer 'yes', 'no' or 'don't know'.
Several key hospital characteristics were considered. Annual birth volume was defined using the self-reported hospital data and categorized as low (<110), medium (111-240), medium-high (241-460) and high (>460). Survey respondents were asked about the types of provider performing obstetric services in their hospitals, and indicator variables for the presence of the following clinician types: OBGYNs, family physicians, CNMs, and general surgeons. Indicators for the most common combinations of clinicians were also included: OBGYNs without family physicians, OBGYNs and family physicians, and family physicians without OBGYNs. Finally, the type of operating room (OR) available for deliveries was reported, and then hospitals were categorized as having a dedicated obstetric OR, a general OR or both.
Analyses
To determine the association between each outcome (EED and VBAC policies) and hospital characteristics (including birth volume, staffing, and OR facilities) Chi-squared and Fisher's exact tests were conducted as appropriate. Analyses were performed using Statistical Analytical Software v9.3 (SAS Institute; http://www.sas.com) and p values <0.05 were considered statistically significant.
Ethics approval
This research was approved by the University of Minnesota Institutional Review Board (ID 1209S20781).
Descriptive information
Participating rural hospitals were split almost evenly across birth volume categories (Table 1). Most hospitals had obstetricians (OBGYNs) attending births (76.6%) and just over half of the hospitals had family physicians attending births (55.3%). Smaller proportions had CNMs (31.6%) and general surgeons (23.4%) attending births. Hospitals in this survey had a combination of clinicians attending births with full details described elsewhere32, and the most common combinations of providers were analyzed (Tables 1-3). A total of 44.7% of hospitals had OBGYNs without family physicians attending deliveries, 23% had family physicians without OBGYNs, and 32.4% had both family physicians and OBGYNs. Over half (55.3%) of the hospitals surveyed had fewer than five clinicians providing obstetric services at their hospital. Most hospitals had a general OR only (58.2%), with only 39.3% of rural hospitals having a dedicated obstetric OR.
Table 1: Hospital birth volume and practitioner characteristics (n=244)
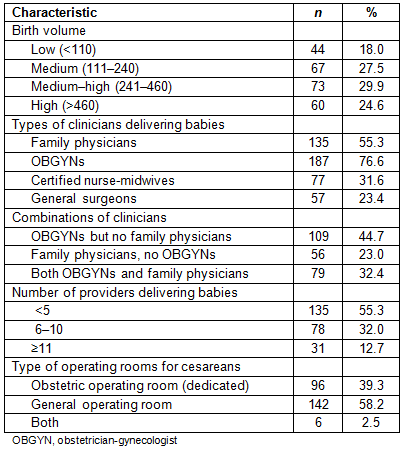
Early elective delivery policies by hospital birth volume and staffing
At the time of the survey, most hospitals had an EED policy in place (89.8%), with the majority having a hard stop policy (70.1%). This means that induction or cesarean without medical indication prior to 39 weeks gestation were prohibited at most hospitals. The medium-high birth volume hospitals were most likely to have a hard stop policy (75.3%). The lowest birth volume category of hospitals had the highest percentage of hospitals with no EED policy (15.9%; Table 2). When comparing hospital staffing and type of EED policy, hospitals with family physicians attending deliveries were more likely to have a hard stop policy than hospitals that did not have family physicians attending deliveries (76.3% vs 62.4%; p=0.05). There were no associations between EED policy type and delivery by obstetricians, CNMs, general surgeons, or a combination of providers. Hospitals with more providers attending deliveries were more likely to have a hard stop policy than those with fewer providers, although this difference was not statistically significant at conventional levels (p=0.07). Finally, hospitals that reported tracking their EED rates were more likely to have a hard stop policy in place (72.6%) than hospitals that did not track (38.9%; p<0.0001). The majority of hospitals (72.7%) reported their EED rates as 0% with very little variation in this outcome, and so the rates were not included in the analyses.
Table 2: Comparison of early elective delivery policy by hospital birth volume and staffing
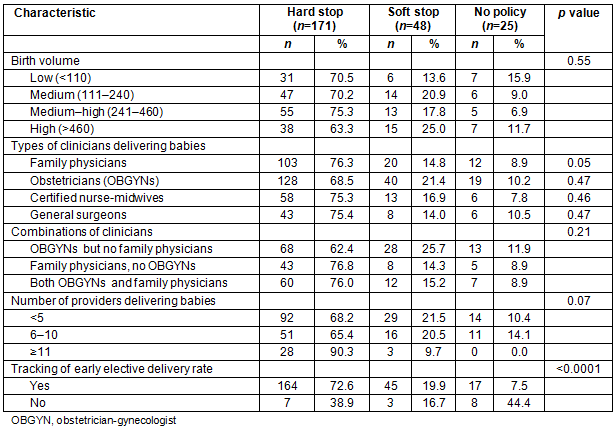
Allowance of vaginal birth after cesarean by hospital birth volume and staffing
Less than half of the rural hospitals surveyed allowed VBAC deliveries (38.1%). Rural hospitals with a higher birth volume were more likely to allow VBAC deliveries (p=0.001; Table 3). The allowance of VBAC deliveries differed significantly by staffing. Hospitals where obstetricians and CNMs attended deliveries were more likely to allow VBAC deliveries than hospitals without these types of clinicians (p=0.01 and p=0.03, respectively). Hospitals where family physicians and general surgeons attended deliveries were less likely to allow VBAC deliveries (p=0.002 and p=0.04, respectively). When analyzing combinations of providers, hospitals with OBGYNs were again more likely than those with family physicians to offer VBAC (p=0.003). There was no difference in VBAC allowance when looking at number of providers per hospital (p=0.18). Also, hospitals with a dedicated obstetric OR were more likely to allow VBAC deliveries (53.1%) compared to those with a general OR (28.2%; p=0.0003).
Table 3: Comparison of allowance of vaginal birth after cesarean by hospital birth volume and staffing
Discussion
This study sought to describe EEDs and VBACs as they differ by staffing, facilities, and birth volume in rural hospitals in the USA. VBAC and EED are distinct, and hospital policies governing their use may depend on different factors related to hospital capacity and workforce, but both VBAC and EED are cornerstones of current quality improvement efforts in maternity care, and post particular challenges in rural settings. Consistent with evidence-based care and professional guidelines20-22, most rural hospitals in this study had an EED policy, and the vast majority had a hard stop policy prohibiting elective deliveries before 39 weeks gestation. There were some important, detectable differences in EED policy across rural hospitals. Hospitals with family physicians attending deliveries were more likely to have a hard stop policy than hospitals that did not have family physicians, and hospitals that reported tracking their EED rates were also more likely to have a hard stop policy in place.
Policy differences across rural hospitals are important, given the differences in morbidity and mortality amongst infants at 37 weeks versus 39 weeks that have been demonstrated in rigorous, well-controlled studies13,18,19,22. However, these differences may occur as a result of the variability across rural maternity hospitals, in terms of staffing, facilities, and capacity. Implementing a hard-stop EED policy in rural hospitals may pose unique challenges given the considerably greater distance patients and providers need to travel to reach the hospital and greater poverty in rural areas, compared with urban areas2,5. Most of the prior research on EED policies focused on successes in urban contexts21, which may not be generalizable to rural areas, and future research may explore the implementation process for EED policies in rural hospitals. Efforts to promote EED policies have now extended to payment decisions, with at least one state Medicaid agency prohibiting reimbursement for EED before 39 weeks gestation13,22. Such a policy is intended to hold the healthcare provider, hospital, and patient accountable to help prevent EEDs, but may create distinct challenges for rural hospitals, where a greater proportion of births are Medicaid-funded1.
This study found that if a rural hospital has family physicians attending births, they are more likely to have a hard stop EED policy in place. Rural hospitals in this survey with a lower volume of births were more likely to have family physicians attending deliveries4. This result was unexpected given that the lower birth volume hospitals where family physicians attend births may have more limited resources and capacity for policy change. However, these results imply the smaller scale may be an advantage in adopting a policy change that consists of a non-intervention (as opposed to a policy requiring greater use of a procedure). In this case, in low-volume settings, there may be fewer obstetric care providers, potentially easing the burden of provider resistance33; in a hospital with more deliveries and therefore more obstetric unit staff, it may be more difficult to implement policy change. Although rural communities face a number of challenges, smaller volume size could be an advantage for obtaining buy-in and assent for changes to clinical management guidelines and hospital policies.
Rural hospitals with higher birth volume, those with obstetricians and CNMs, and those with a dedicated obstetric OR were all statistically significantly more likely to allow VBAC deliveries. Those rural hospitals where family physicians and general surgeons delivered babies were statistically significantly less likely to allow VBACs. These findings may all flow from a relationship between hospital birth volume, resources and staffing, as they relate to recommendations for safely offering VBAC27. Professional guidelines on the use of VBAC require hospitals to have dedicated OR staff, anesthesia, and pediatrics immediately available for an emergency cesarean, which some low volume hospitals in rural areas may be unable to consistently provide. Because of this, in some smaller rural hospitals, TOLAC is no longer offered27. Enforcement of VBAC guidelines may limit women's access to TOLAC in rural areas, but the guidelines are based on expert opinion regarding patient safety for women with a prior cesarean. The findings reflect the broader literature, indicating that some rural hospitals just do not have the patient volume, facilities, or staff resources to sustain a dedicated OR, dedicated anesthesia, and dedicated OR staff around the clock, every day11.
The 2010 NIH consensus statement on VBAC does not clearly identify or discuss geographic disparities in access to TOLAC30, nor has the literature explored the crucial balance between patient safety and ensuring access to maternity care for women in rural areas2. To date, published findings on the safety of TOLAC draw on data from primarily urban teaching hospitals, settings that have the patient volume and resources to ensure capacity of performing immediate emergency cesarean deliveries30. While rapid availability of cesarean delivery for women undergoing TOLAC is clearly justified, the specific parameters of 'immediate availability' allow little flexibility for rural clinicians and hospitals, and comparative data examining in detail the effects of 'decision to incision' timing in rural contexts are not available to inform decisions34. In fact, the American Academy of Family Physicians guidelines differ from those issued by ACOG, stating that TOLAC should not be restricted only to facilities with available surgical teams present throughout labor because there is no evidence that these additional resources result in improved outcomes35. At the same time, it is clinically appropriate that a management plan for uterine rupture and other potential emergencies requiring cesarean section should be documented for each woman undergoing TOLAC35. More research is needed in rural contexts to inform both hospital policies and patient decisions about VBAC.
Women living in rural areas where VBAC is not offered face repeat cesarean as a near certainty, and the health risks of the procedure increase substantially for subsequent cesareans36. The only viable alternative for a rural woman seeking TOLAC would be to travel, potentially great distances or across state lines, to seek care in a facility that offers VBAC, which may require substantial personal, financial, and social resources and result in socioeconomic disparities in access. Pregnant women who live in rural areas and have had a prior cesarean face limited options, and a dearth of information to fully inform their decisions. They also receive care from a range of different types of clinicians, whose professional guidelines differ with respect to VBAC. There is a need for rural-specific discussions among the professional associations representing the clinicians who provider maternity care in rural areas (OBGYNs, midwives, family physicians) to establish consensus regarding the risks and benefits of VBAC in low-volume and rural areas. Decision aids, including internet-based models, have been successfully employed, and could be tailored to address the particular concerns of rural women and clinicians37.
This study presents the first rural-specific data on hospital policies regarding EED and VBAC, two areas of focus of recent maternity care quality efforts, but it is not without limitations. Results were based on a telephone survey to a single responder, and there may be variability across hospitals in the respondent's knowledge of the hospital's obstetrical policies and practices. The specific content of each hospital's EED and VBAC policies was not assessed in the survey, so the authors were not able to assess the language used or the enforcement mechanisms that may have been specified by particular hospitals. Future qualitative work may uncover more granular information about policy formulation and content. Information is self-reported by hospitals, and thus may be subject to social desirability bias, which, in this case, would have biased results toward the null hypothesis if all respondents were influenced by similar norms. The survey data did not include information on planned versus unplanned VBACs, which would be an interesting measure to analyze in rural contexts. While hospital EED rates were collected, the majority of the hospitals reported 0% so there is very little variation in this outcome. Given the small number of non-zero responses, the analysis of this rate as an outcome was not conducted. Rural areas are diverse, and rural hospitals vary widely across states and regions, and the results based on these nine states may not be fully generalizable to all rural areas of the USA. However, the response rate for this survey was very high (86%), and the rural hospitals surveyed in this study were similar to all rural hospitals with obstetric units as reported in the American Hospital Association Annual Survey4. While this analysis focused on policies, in an effort to inform hospital administration and leadership in quality improvement activities, future research should also examine actual clinical practices regarding EED and VBAC in rural communities.
This study examined policies for EED and VBAC in rural maternity hospitals in nine US states. Most rural hospitals had a hard stop policy, explicitly forbidding EED, consistent with national recommendations. VBAC access is limited in rural areas. Rural hospitals with higher birth volume, those with obstetricians and CNMs, and those with a dedicated obstetric OR were all more likely to allow VBAC deliveries. Where possible, feasible and safe, policies such as the hard stop policy for EEDs and VBAC allowance are encouraged to best serve rural women and to reflect evidence-based practices. Rural areas are unique as distance to delivery is associated with increased risk of poor outcomes, and the need for nearby access to high quality obstetric care has been well documented, particularly in Canada, but is relevant across rural settings internationally9,10 General guidelines and recommendations in obstetrics ought to account for variability in implementation capacity across rural maternity hospitals.
Acknowledgements
The authors acknowledge helpful input from Dr Kozhimannil's Rural Hospital Obstetric Advisory Group; the rural hospital survey respondents; the Office of Measurement Services at the University of Minnesota, for fielding the survey; and Michelle Casey, for her helpful review, insights and suggestions.
References
1. Kozhimannil KB, Hung P, Prasad S, Casey M, Moscovice I. Rural-urban differences in obstetric care, 2002-2010, and implications for the future. Medical Care 2014; 52: 4-9. http://dx.doi.org/10.1097/MLR.0000000000000016
2. Health disparities in rural women. Committee opinion no. 586. American College of Obstetricians and Gynecologists. Obstetrics & Gynecology 2014; 123: 384-388. http://dx.doi.org/10.1097/01.AOG.0000443278.06393.d6
3. Rayburn WF, Klagholz JC, Murray-Krezan C, Dowell LE, Strunk AL. Distribution of American Congress of Obstetricians and Gynecologists fellows and junior fellows in practice in the United States. Obstetrics & Gynecology 2012; 119: 1017-1022. http://dx.doi.org/10.1097/AOG.0b013e31824cfe50
4. Kozhimannil KB, Casey MM, Hung P, Han X, Prasad S, Moscovice IS. The rural obstetric workforce in US hospitals: challenges and opportunities. Journal of Rural Health 2015; 31(4): 365-372. http://dx.doi.org/10.1111/jrh.12112
5. Hart LG, Larson EH, Lishner DM. Rural definition for health policy and research. American Journal of Public Health 2005; 25: 1149-1155. http://dx.doi.org/10.2105/AJPH.2004.042432
6. Agency for Healthcare Research and Quality. 2012 national healthcare disparities report. (Internet) 2013. Available: http://archive.ahrq.gov/research/findings/nhqrdr/nhdr12/index.html (Accessed 7 September 2015).
7. Hung P, Kozhimannil KB, Casey M, Moscovice IS. Why are obstetric units in rural hospitals closing their doors? Health Services Research 2016; 51: 1546-1560.
8. Simpson KR. An overview of distribution of births in United States hospitals in 2008 with implications for small volume perinatal units in rural hospitals. Journal of Obstetrics, Gynecology, and Neonatal Nursing 2011; 40: 432-438. http://dx.doi.org/10.1111/j.1552-6909.2011.01262.x
9. Grzybowski S, Fahey J, Lai B, Zhang S, Aelicks N, Leung B, et al. The Safety of Canadian rural maternity services: a multi-jurisdictional cohort analysis. BMC Health Services Research 2015; 15: 410. http://dx.doi.org/10.1186/s12913-015-1034-6
10. Grzybowski S, Stoll K, Kornelsen J. Distance matters: a population based study examining access to maternity services for rural women. BMC Health Services Research 2011; 11: 147. http://dx.doi.org/10.1186/1472-6963-11-147
11. Kozhimannil KB, Hung P, Prasad S, Casey M, McClellan M, Moscovice IS. Birth volume and the quality of obstetric care in rural hospitals. Journal of Rural Health 2014; 30(4): 335-343. http://dx.doi.org/10.1111/jrh.12061
12. Snowden JM, Cheng YW, Emeis CL, Caughey AB. The impact of hospital obstetric volume on maternal outcomes in term, non-low-birthweight pregnancies. American Journal of Obstetrics and Gynecology 2015; 212(3): 380.e1-9. http://dx.doi.org/10.1016/j.ajog.2014.09.026
13. Nonmedically indicated early-term deliveries. Committee opinion No. 561. American College of Obstetricians and Gynecologists. Obstetrics & Gynecology 2013; 121: 911-915. http://dx.doi.org/10.1097/01.AOG.0000428649.57622.a7
14. Caughey AB, Sundaram V, Kaimal AJ, Cheng YW, Gienger A, Little S, et al. Maternal and neonatal outcomes of elective induction of labor. Evidence Report/Technology Assessment (Full Report) (Internet) 2009; 176: 1-257. Available: http://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0007568/ (Accessed 7 September 2015).
15. Rayburn WF, Zhang J. Rising rates of labor induction: present concerns and future strategies. Obstetrics & Gynecology 2002; 100: 164-167. http://dx.doi.org/10.1097/00006250-200207000-00024
16. Zhang J, Yancey MK, Henderson CE. US national trends in labor induction, 1989-1998. Journal of Reproductive Medicine 2002; 47: 120-124. http://dx.doi.org/10.1097/00006254-200208000-00011
17. Medically indicated late-preterm and early-term deliveries. Committee opinion no. 560. American College of Obstetricians and Gynecologists. Obstetrics & Gynecology 2013; 121: 908-910. http://dx.doi.org/10.1097/01.AOG.0000428648.75548.00
18. Clark SL, Miller DD, Belfort MA, Dildy GA, Frye DK, Meyers JA. Neonatal and maternal outcomes associated with elective term delivery. American Journal of Obstetrics and Gynecology 2009; 156.e1-156.e4.
19. Hibbard JU, Wilkins I, Sun L, Gregory K, Haberman S, Hoffman M, et al. Consortium on safe labor, respiratory morbidity in late preterm births. Journal of American Medical Association 2010; 304: 419-425. http://dx.doi.org/10.1001/jama.2010.1015
20. Clark SL, Frye DR, Meyers JA, Belfort MA, Gary AD, Kofford S, et al. Reduction in elective delivery at >39 weeks of gestation: comparative effectiveness of 3 approaches to change and the impact on neonatal intensive care admission and stillbirth. American Journal of Obstetrics and Gynecology 2010; 203(5): 449e1-6. http://dx.doi.org/10.1016/j.ajog.2010.05.036
21. Oshiro BT, Kowalewski L, Sappenfield W, Alter CC, Bettegowda, VR, Russell R, et al. A multistate quality improvement program to decrease elective deliveries before 39 weeks of gestation. Obstetrics & Gynecology 2013; 121: 1025-1031. http://dx.doi.org/10.1097/AOG.0b013e31828ca096
22. Fowler TT, Schiff J, Applegate MS, Griffith K, Fairbrother GL. Early elective deliveries accounted for nearly 9 percent of births paid for by Medicaid. Health Affairs 2014; 33(12): 2170-2178. http://dx.doi.org/10.1377/hlthaff.2014.0534
23. Hamilton BE, Hoyert DL, Martin JA, Strobino DM, Guyer B. Annual summary of vital statistics: 2010-2011. Pediatrics 2013; 131: 548-558. http://dx.doi.org/10.1542/peds.2012-3769
24. Martin JA, Hamilton BE, Ventura SJ, Osterman MJ, Mathews TJ. Births: final data for 2011. National Vital Statistics Report 2013; 62(2): 1-90.
25. Centers for Disease Control and Prevention. Rates of cesarean delivery - United States, 1991. Morbidity and Mortality Weekly Report 1993; 42: 285-289.
26. Spong YS, Berghella V, Mercer M, Saade GR. Preventing the first cesarean delivery: summary of a Joint Eunice Kennedy Shriver National Institute of Child Health and Human Development, Society for Maternal-Fetal Medicine, and American College of Obstetricians and Gynecologists Workshop. Obstetrics & Gynecology 2012; 120(5): 1181-1193.
27. Vaginal birth after previous cesarean delivery. Practice Bulletin No. 115. American College of Obstetricians and Gynecologists. Obstetrics & Gynecology 2010; 116: 450-463. http://dx.doi.org/10.1097/AOG.0b013e3181eeb251
28. Leeman LM, Beagle M, Espey E, Ogburn T, Skipper B. Diminishing availability of trial of labor after cesarean delivery in New Mexico hospitals. Obstetrics & Gynecology 2013; 122: 242-247. http://dx.doi.org/10.1097/AOG.0b013e31829bd0a0
29. Korst LM, Gregory KD, Fridman M, Phelan JP. Nonclinical factors affecting women's access to trial of labor after cesarean delivery. Clinical Perinatology 2011; 38(2): 193-216. http://dx.doi.org/10.1016/j.clp.2011.03.004
30. National Institutes of Health Consensus Development Conference: vaginal birth after cesarean: new insights. Obstetrics & Gynecology 2010; 115(6): 1279-1295. http://dx.doi.org/10.1097/AOG.0b013e3181e459e5
31. US Department of Health and Human Services, Office of Disease Prevention and Health Promotion. Healthy people 2020. (Internet). Available: https://www.healthypeople.gov/2020/topics-objectives/topic/maternal-infant-and-child-health/objectives (Accessed 8 September 2015).
32. Kozhimannil K, Casey M, Hung P, Prasad S, Moscovice I. The obstetric care workforce in critical access hospitals (CAHs) and rural non-CAHs policy brief. (Internet) 2014. Available: http://rhrc.umn.edu/wp-content/files_mf/obworkforcepolicybrief.pdf (Accessed 7 January 2015).
33. Cabana MD, Rand CS, Powe NR, Wu AW, Wilson MH, Abboud PA, et al. Why don't physicians follow clinical practice guidelines? A framework for improvement. Journal of American Medical Association 1999; 282(15): 1458-1465. http://dx.doi.org/10.1001/jama.282.15.1458
34. Smith GC, Pell JP, Pasupathy D, Dobbie R. Factors predisposing to perinatal death related to uterine rupture during attempted vaginal birth after caesarean section: retrospective cohort study. British Medical Journal 2004; 329: 375. http://dx.doi.org/10.1136/bmj.38160.634352.55
35. Wall E, Roberts R, Deutchman M, Hueston W, Atwood L, Ireland B. Trial of labor after cesarean (TOLAC), formerly trial of labor versus elective repeat cesarean section for the woman with a previous cesarean section. Annals of Family Medicine. (Internet) 2005. Available: http://www.annfammed.org/cgi/content/full/3/4/378/DC1 (Accessed 7 September 2015).
36. Silver RM, Landon MB, Rouse DJ, Leveno K, Spong CY, Thom E, et al. Maternal morbidity associated with multiple repeat cesarean deliveries. Obstetrics & Gynecology 2006; 107: 1226-1232. http://dx.doi.org/10.1097/01.AOG.0000219750.79480.84
37. Shorten A, Fagerlin A, Illuzzi J, Kennedy H, Lakehomer H, Pettker C, et al. Developing an internet-based decision aid for women choosing between vaginal birth after cesarean and planned repeat cesarean. Journal of Midwifery and Women's Health 2015; 60(4): 390-400. http://dx.doi.org/10.1111/jmwh.12298


