Introduction
Antimicrobial resistance (AMR) has been recognised as an urgent health priority, both nationally and internationally1,2. A key contributor to the development of AMR is antimicrobial use. Australia has a high per capita consumption of antimicrobials3, and data suggest that much of this antimicrobial use is inappropriate or unnecessary4. The release of the World Health Organization’s Global action plan on antimicrobial resistance2 and Australia’s first national antimicrobial resistance strategy 2015–20195 in 2015 stressed the need for action to improve the use of antimicrobials in all sectors, including in humans, animals and ecosystems.
Antimicrobial stewardship (AMS) is defined as ‘a collective set of strategies to improve the appropriateness and minimise the adverse effects of antibiotic use, including resistance, toxicity and costs’6. All Australian hospitals are now required to show evidence of AMS activities as part of the National Safety and Quality Health Service (NSQHS) accreditation7. A similar requirement exists in some other countries (eg Canada)8. The essential elements of an AMS program as described in Antimicrobial Stewardship in Australian hospitals9 are:
- clinical guidelines that are tailored for local antimicrobial susceptibility patterns
- formulary restriction and approval systems
- regular review and feedback on antimicrobial prescribing to individual prescribers
- monitoring of overall antimicrobial prescribing
- selective reporting of susceptibility testing results.
Studies have indicated that Australian regional and rural hospitals are less advanced in their uptake of AMS activities than metropolitan hospitals10,11, and similar themes arise in the Australian and international literature regarding barriers to the uptake of AMS activities in regional, rural and remote hospitals.
Table 1 highlights that lack of access to staff who are skilled in AMS (infectious diseases (ID) physician, pharmacist or clinical microbiologist) is a frequently cited barrier10,12-17. (Other barriers to and enablers of AMS implementation in regional, rural and remote hospitals identified in published studies are summarised in Table 110,12-22.) This suggests a common perception that a hospital needs to have ID/microbiology experts on site to lead AMS programs. Such a view is no surprise given that early AMS literature came almost exclusively from tertiary hospitals with access to such resources23. Strongly linked to this is the reported barrier of a lack of training and education in infectious diseases and AMS for other hospital staff10.
Recruitment of healthcare staff is often a problem in rural areas. In one study, a lack of pharmacy resources was reported as a barrier to AMS in 82% of regional and rural hospitals10.
AMS programs that address some of these common barriers to AMS program implementation in regional, rural and remote hospitals are described in this review. These programs highlight key considerations that may assist Australian regional, rural and remote hospitals to implement, sustain or improve their AMS programs.
Table 1: Barriers and enablers to antimicrobial stewardship implementation in regional, rural and remote hospitals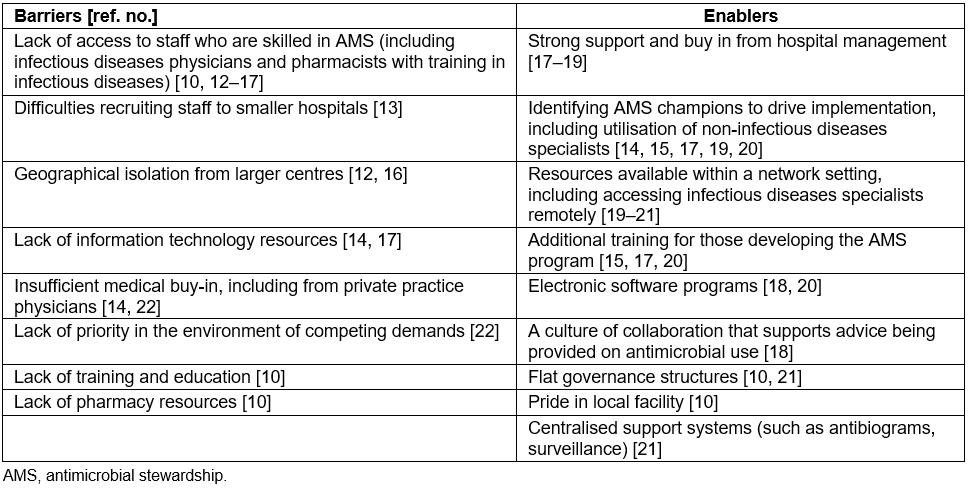
Methods
A literature search was undertaken on 1 September 2017 in the following databases: Ovid Medline (1946 to present), Scopus (1995 to present) and Web of Science (1900 to present). The date of publication was restricted to 1997–current, reflecting the newness of the topic. There were no restrictions on study design, study location, journal or publication type. Non-English language articles were retained with the intention of seeking translation if pivotal for the review. A scan of the reference list of included articles was undertaken to identify any articles not captured in the original search (cited search). The ‘cited by’ listings in Scopus were also reviewed to identify any additional articles. A grey literature search (Trove, ProQuest Dissertations and Theses Global, internet) was also undertaken.
The search terms are shown in Figure 1.
Articles were included if they described an AMS program in the regional, rural or remote hospital setting that had applicability for the Australian context. For example, hospitals greater than 300 beds that were described as ‘community’ were excluded as regional hospitals in Australia rarely exceed that size.
A narrative review was conducted to describe models from heterogeneous qualitative, quantitative and guideline resources.
 Figure 1: Search terms included in the study.
Figure 1: Search terms included in the study.
Results
The Ovid Medline search identified 432 articles, of which 379 were immediately excluded based on their descriptive title. Abstracts and full text were reviewed for the remaining 53 articles, from which 35 were subsequently excluded. An additional five references were located by scanning the reference list of included articles and through the grey literature. After accounting for duplicate citations, no additional articles suitable for inclusion were identified through Scopus or Web of Science searches. No additional articles were identified through a ‘cited by’ search. A further five articles were excluded but four were included as a supporting reference. Figure 2 shows an overview of the search results.
Eighteen published articles were deemed relevant to this review.
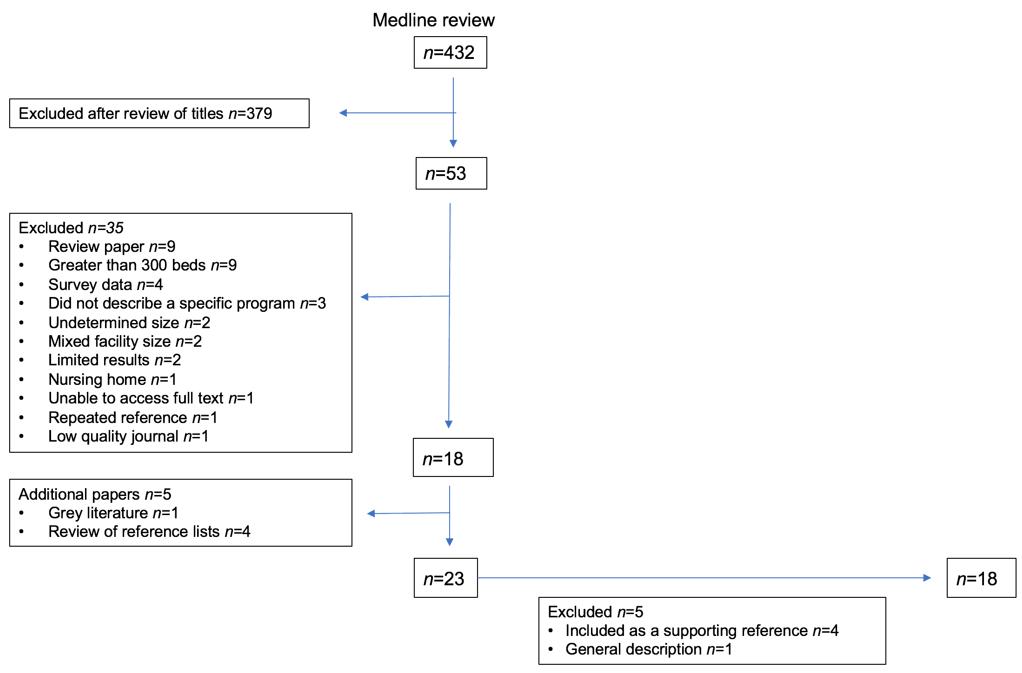 Figure 2: Overview of search results.
Figure 2: Overview of search results.
General guidance for regional, rural and remote hospitals
The most recent guidance on implementing AMS programs in small and critical access hospitals was released by the Centers for Disease Control and Prevention (CDC) in mid-201724. This publication provides a range of practical implementation options that might be useful in meeting the CDC’s core elements for AMS programs. The Australian Commission on Safety and Quality in Healthcare produces guides for NSQHS accreditation7. Both their full guide and their guide for small hospitals outline the evidence requirements for AMS activities. The Safety and quality improvement guide for Standard 3: Preventing and Controlling Healthcare Associated Infections (October 2012) lists a number of strategies that non-urban and non-tertiary facilities could adopt25. In 2016, the Pew Trust released A path to better antibiotic stewardship in inpatient settings26. It included a number of case studies of AMS program implementation, including examples from regional and smaller hospitals. It outlined the structure of the program, sustainability and key learnings.
Published literature
The AMS initiatives described in published articles are summarised in Table 2, including the number of citations for each.
These initiatives were categorised into groups that address different issues relating to AMS program delivery in regional, rural and remote hospitals. The initiatives are not mutually exclusive and more than one could be employed by any given health service.
Table 2: Summary of articles included in the review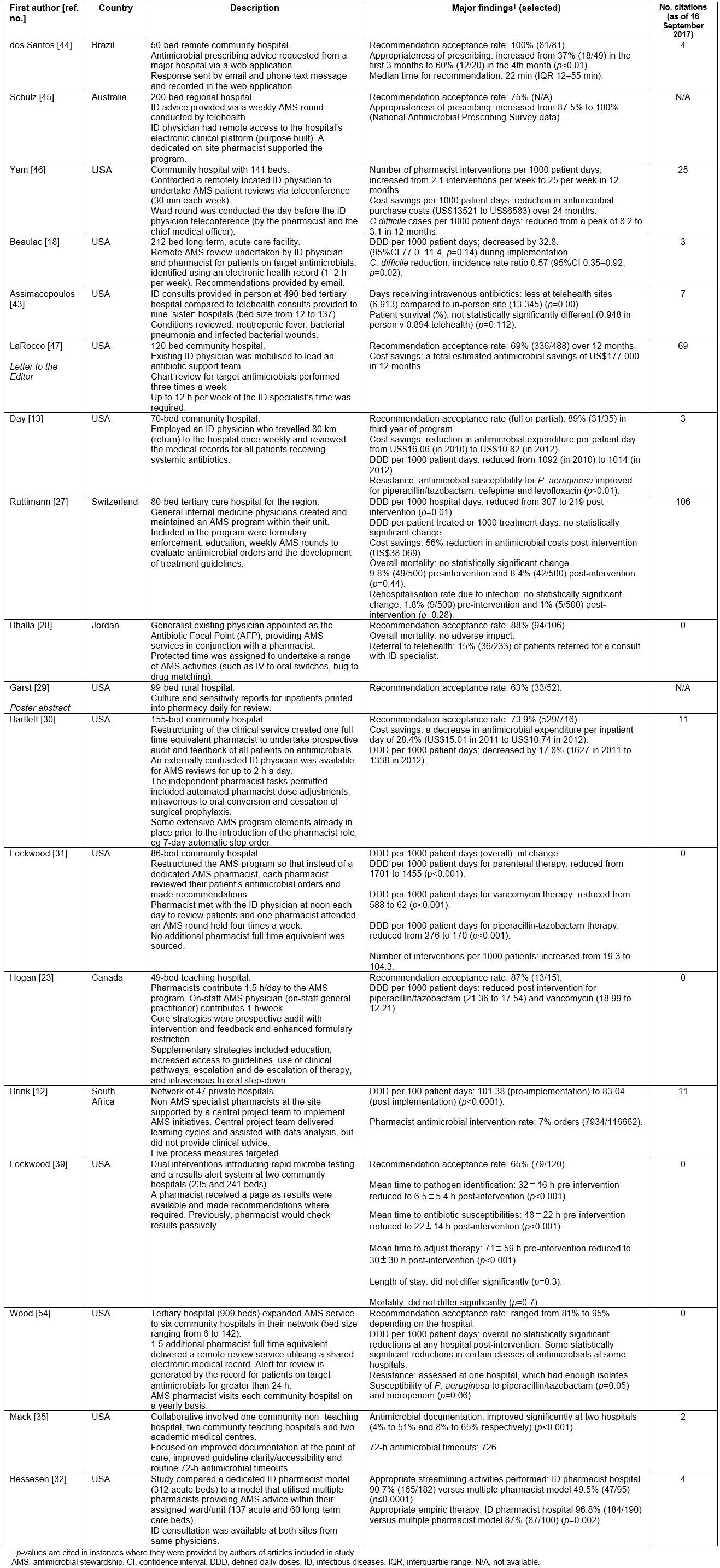
Enabling hospital staff to conduct AMS activities in the absence of on-site ID experts: These initiatives include programs provided by doctors who are not ID or clinical microbiology specialists, pharmacist-led programs, externally led programs, nurse-led programs and technology.
Programs with AMS activities provided by doctors who are not ID or clinical microbiology specialists Where ID specialists and clinical microbiologists are not available, it has been suggested that non-ID trained medical staff could manage an AMS program15.
An 80-bed hospital in Switzerland described utilising general internal medicine physicians to create and maintain an AMS program27. The program was considered successful based on a reduction in overall antimicrobial consumption and antimicrobial costs at the hospital27. Improvements in the quality of prescribing were not described.
The Medicines San Frontiers hospital in Jordan spearheaded its AMS program with a designated ‘antibiotic focal point’ physician28. The physician was rewarded financially for undertaking the role, but no additional human resources were appointed28. Importantly, telemedicine was available for complex consultations requiring ID specialist input. A recommendation acceptance rate of 88% (94/106) was achieved and there was an associated reduction in antimicrobial costs28.
Pharmacist-led programs In the absence of a medical-led AMS program, pharmacists have been described as an alternative lead for AMS activities25.
An AMS program that utilised non-ID trained pharmacists was described at a 99-bed US hospital29. This study illustrated a single intervention where culture and susceptibility reports for specimens submitted for microbiology testing were routinely sent to the pharmacy each day for pharmacist review. The pharmacist then offered advice on antibiotic prescribing to the treating doctor. The reported recommendation acceptance rate was 63% (33/52)29. There was limited reported impact on the pharmacists’ daily workload (estimated at less than 10 min per day)29.
An AMS model in a 155-bed US facility reported on the rotation of the AMS role weekly through a group of pharmacists (clinical and dispensary-trained)30. The AMS activities performed included automated pharmacist dose adjustments and routine cessation of surgical prophylaxis according to agreed guidelines. The authors believed that a shared model of responsibility for AMS made it more sustainable and increased AMS capacity30. Greater job satisfaction and improved knowledge of antimicrobials led to additional interventions outside of the formal AMS program30.
Similarly, an 86-bed US community hospital described a switch from a dedicated AMS pharmacist model to a shared responsibility model that involved their clinical pharmacists31. The clinical pharmacists reviewed their patients’ antimicrobials and recommended interventions where required. A pharmacist attended the AMS round four times a week and all clinical pharmacists met with the ID physician at noon each day to discuss patient cases. No additional pharmacy resources were sourced. Comparing the approaches, there was an increase in interventions after the change in program structure (19.3 and 104.3 interventions per 1000 patients pre- and post-program change, respectively)31. There was a statistically significant reduction in defined daily doses per 1000 patient days for parenteral therapies and certain targeted antimicrobials (vancomycin and piperacillin-tazobactam)31.
A pharmacist-led AMS program described in a 49-bed Canadian hospital introduced several strategies such as prospective audit with intervention and feedback, and enhanced formulary restriction23. It also had a range of supplementary strategies such as the implementation of clinical pathways and de-escalation programs. There was a high recommendation acceptance rate of 87% (13/15) for the prescriber feedback system; however, the number of interventions reported was small23. Reductions in antimicrobial usage were described 23. A core limitation was that the comprehensive program appeared to have been sustained largely by unpaid overtime23.
Pharmacists performing antimicrobial interventions as a part of routine care have also been described32-34. A Canadian study reported that in the absence of a formal AMS program, pharmacists made on average three AMS interventions per antimicrobial treatment course as part of routine pharmaceutical care33. Another study reported that routine AMS interventions by pharmacists could be increased from 1.6 interventions per 1000 patient days to 2.6 interventions per 1000 patient days (p<0.0001) by using clinical surveillance software to identify opportunities for intervention33. Programs have also been described that rely on the services of pharmacy students35.
Externally led programs Some articles describe situations where AMS interventions were led by larger groups external to the hospital.
The US Institute for Healthcare Improvement and the CDC Collaborative developed a program that focused on embedding AMS into the daily workflow of their clinicians15,36. The three target areas were36:
- improved documentation/visibility at points of care (eg antimicrobial durations, indications)
- 72-h antimicrobial timeouts to routinely reassess antimicrobial use
- improved guideline clarity and accessibility.
Access to program details and outcome results across the five hospitals involved in implementing this program is, however, limited.
A large program to support AMS in 47 South African private hospitals has been described12. This program utilised a centralised project centre that provided support to existing pharmacists working within each facility (termed ‘AMS champions’). The central project team provided initial face-to-face training, delivered learning cycles every 6–8 weeks, and provided data analysis and reporting for each site12. The project team did not provide clinical advice regarding individual patient care. A statistically significant reduction in antibiotic consumption between the pre-implementation and post-implementation phases of this project was reported12. No measures of the impact on the appropriateness of prescribing or patient outcomes were provided.
The Duke Antimicrobial Stewardship Outreach Network (DASON) is another example of an externally led program. It is based on the Duke Infection Control Outreach Network program that was developed to support sites to reduce hospital associated infections37. Stewardship pharmacist liaisons are at the core of the program, with oversight from ID physicians. Each stewardship pharmacist liaison provides support for up to eight hospitals. This includes monthly site visits, assistance with data analysis, the preparation of site reports based on the patient-level dosing information on antimicrobials that is uploaded into the DASON portal from each site, and education38. The impact of DASON is yet to be published.
Nurse-led models To date, the literature has not described nurse-led AMS models, although there is an awareness that many infection prevention nurses are playing pivotal roles in establishing and maintaining AMS programs in smaller hospitals25,39. There are now calls for nurses to be formally represented on AMS teams and for greater antimicrobial education for nurses/nurse practitioners39.
Technology Technology, such as rapid diagnostics, has the potential to support the appropriate use of antimicrobials in regional, rural and remote hospitals by ensuring timely treatment. Rapid diagnostic testing (MALDI-TOF) in conjunction with an AMS program was described in two community hospitals located in the US (235 and 241 beds respectively)40. In this program, there were two concurrent interventions. The MALDI-TOF diagnostic was used to identify microbes, and a process was introduced whereby the on-call pharmacist received a page when the diagnostic result was available. The study reported reduced time to pathogen identification and antibiotic susceptibility results being released (p<0.001)40. Pharmacist interventions were accepted more frequently (p<0.001) and time to therapy adjustment was decreased from 71 h to 30 h (p<0.001)40. Mortality rates were reduced from 9.4% (14/132) pre-intervention to 4.9% (11/214) post intervention (p=0.07)40.
Addressing the problem of access to expert ID clinical advice when required: Access to expert ID advice when required for clinical management of individual patients remains important. Pharmacists, infection prevention practitioners and doctors will likely encounter situations in which they need specialist help. Regional, rural and remote hospitals need to find ways to provide reliable access to expert ID advice, and the Safety and quality improvement guide for Standard 3: Preventing and Controlling Healthcare Associated Infections describes telehealth, onsite visits and networks as potential ways of achieving this external support25.
Telehealth Telehealth has been defined as the ‘provision of healthcare remotely by means of a variety of telecommunication tools, including telephones, smart phones and mobile wireless devices (with or without video connection)’41. Telehealth has increased access to specialist care/advice for populations in rural areas41,42. Recently, the Infectious Diseases Society of America released a position statement on telehealth for infectious diseases practice. It concluded that telehealth could assist resource-limited hospitals to undertake more efficient and flexible AMS activities43.
Several articles explore the role of telehealth in supporting AMS in regional, rural or remote hospitals28,44-47. These programs vary in their structure, and human and information technology resource requirements.
In a Brazilian telehealth model, general practitioners from a 50-bed remote hospital utilised an electronic portal to receive specialist clinical advice on antimicrobial choice at an average turnaround time of 22 min45. The model appeared to function with no additional AMS human resources at the remote hospital. In contrast, the telehealth model described by Yam et al47 utilised AMS human resources at the hospital to ‘pre-review’ patients before presentation to the ID specialist during the telehealth consultation.
A retrospective review of the outcomes of a telehealth AMS model compared to an on-site model was published in 200844. The study showed that there were no statistically significant differences in patient survival or inter-hospital transfer rates between the two models44. It provides some evidence that telehealth provision of an ID consult may be able to provide similar outcomes to a face-to-face ID consult. The authors acknowledged that not all clinical differences between patients were accounted for (such as comorbid conditions), and this may have influenced the evaluation.
Visiting ID specialist A number of smaller hospitals have recruited ID specialists to provide AMS services48-53. In some instances, an ID specialist may be employed to provide regular site visits to see patients requiring consultation (eg once per week). A study at a 70-bed community hospital in the US described such a ‘fly-in, fly-out’ (FIFO) arrangement13. High recommendation acceptance rates were reported (89%, 31/35), and the financial savings were described as significant enough to cover the cost of the FIFO specialist13.
Network or ‘hub and spoke’ models A network or ‘hub and spoke’ model comprises a central facility (hub) that manages more complex care, and satellite (spoke) facilities that deliver less complicated care54. The spokes are often located in regional areas54.
A ‘hub’ hospital in the US expanded its AMS program to six community hospitals within their network which previously had no access to an ID consultation service55. Patients on a restricted/controlled antimicrobial for greater than 24 h were identified in the shared electronic record, triggering a remote chart review by the AMS pharmacist55. Access to an ID specialist for clinical advice on individual patient management was available. Overall, total antimicrobial use did not significantly change as a result of the program; however, the impact varied between different hospitals55. There was no description of clinical outcomes in the study.
Discussion
This review highlights that there are several viable models for AMS programs that regional, rural and remote hospitals could explore to address the existing difficulties with establishing and maintaining such services. These include the development of non-specialist led AMS services and strategies to access remote expert support when required.
The introduction of pharmacist-led models, although one of the more frequently described models in the literature, is likely to be limited in many facilities by the lack of pharmacy resources. Pharmacist-led models without additional pharmacist resources can lead to increased workload and unpaid overtime for staff involved23, although less comprehensive programs and more manageable programs are also possible29. Whilst general pharmacist staffing is outlined in published ratios such as those provided by the Society of Hospital Pharmacists of Australia56, an additional ‘AMS loading’ may be required to support AMS functions in regional, rural and remote hospitals if pharmacists are to take a lead role. There would also need to be robust evaluation of the quality of the pharmacist recommendations in terms of the clinical appropriateness of the advice given. This has not been described in the published articles reviewed and is a key patient safety issue. The support of an external project team may help to enable local pharmacists to play a more active role as described in the South African model12.
Access to specialist ID advice is an important consideration for regional, rural or remote hospitals. Whilst it may be possible to conduct an AMS program in the absence of qualified ID specialists, there will be situations where expert advice is required. A FIFO ID specialist to provide expert advice might be achievable for some remote Australian hospitals. Physically attending a site may be more beneficial compared to a remote review model (such as those utilising an electronic health record) as it could enable a better appreciation of issues that might not otherwise be apparent if reviewing a patient remotely. Clearly, a FIFO model must incorporate a regular agreed service to ensure continuity. The timeliness of access to advice may be an issue, as a single specialist is unlikely to be able to provide continuous on-call assistance. For this reason, teams of specialists may be able to coordinate on-call arrangements to cover clusters of smaller facilities in a rotating roster.
A number of Australian hospitals currently access specialist ID advice through informal networks. Formalised clinical partnerships with larger hospitals is a preferable general approach to better support rural hospitals57. Formalisation of the arrangement will increase accountability on each side of the consultation, both for the quality of the information provided and the advice received. Regular contact with a consistent group of experts can build a rapport between clinicians at rural and metropolitan centres. Clinical partnerships also foster the sharing of resources so that small hospitals don’t need to develop their own tools but can use those from larger hospitals in the network. Similarly, such tools could also be developed and shared by external groups.
There is a lack of guidance for regional, rural and remote hospitals on staffing requirements for AMS programs. A recent commentary highlighted that few countries have recommended staffing ratios58, and these have not been specifically described for regional, rural or remote hospitals. This is certainly an area for further research.
Information technology can be an enabler to increase access to expert advice for regional, rural and remote hospitals10,21. Electronic medication management systems in hospitals can provide time-saving automated data mining to help identify patients on target antimicrobials. Multi-site computerised clinical decision support systems (CDSS) for AMS have been evaluated. In the study by Bond et al, the impact of the centrally deployed CDSS was evaluated in five of the twelve hospitals utilising it within a network59. The evaluation included two regional hospitals. The interrupted time series study showed a reduction in usage of target antimicrobials and antimicrobial expenditure59. The authors concluded that the ‘multi-site approach allowed for collective interventions to be employed with reduced workload at individual hospital sites’59. This warrants further exploration for regional, rural and remote hospitals.
To date, information about more broadly coordinated approaches to AMS in regional, rural and remote hospitals (eg at jurisdictional levels) has been limited in the published literature. Apart from examples of network (‘hub and spoke’) models, the published articles report programs implemented and evaluated at single sites. Examples of national initiatives (such as the national antibiotic stewardship intervention in Scotland to target four antimicrobials known to increase the risk of C. difficile infection)60 have not specifically addressed regional or rural issues. Whilst the CDC’s Implementation of antibiotic stewardship core elements at small and critical access hospitals24 provides practical options for smaller hospitals, the advice is based on discussions and case studies only. A national free AMS mentoring program in the USA reported mixed levels of impact and issues with loss of momentum over time, but did provide an interesting model to consider61. Within Australia, the Statewide Antimicrobial Stewardship program funded by Queensland Health is an example of a more coordinated approach to AMS program delivery in regional, rural and remote hospitals21. The Clinical Excellence Commission also provides centralised support to regional, rural and remote hospitals in New South Wales. Descriptions of the activities and impact of such services are awaited with interest.
There would certainly seem to be some areas where a coordinated approach over a large region would be beneficial to avoid duplication of effort and minimise cost. For example, at present in Australia, CDSS programs must be purchased or an equivalent developed in house. The establishment of a jurisdictional licence for these programs would be a positive step towards improving access.
As varied AMS models are being established, it is important to ensure that they are safe. As such, some measure of the appropriateness of prescribing is needed to regularly monitor and compare the success of programs. While clinical outcomes would be useful to monitor, they are multifactorial, and it is difficult to attribute mortality to the AMS intervention alone. A standardised audit tool measuring appropriateness of antimicrobial prescribing (such as the Australian National Antimicrobial Prescribing Survey) would be a reasonable existing surrogate to monitor the quality of care provided, regardless of the model in place at that hospital. Work has been done to show that auditors at smaller hospitals and rural hospitals can be trained to assess prescriptions in a consistent way to allow comparability of data62.
The issues faced by AMS programs are not unique and the successes and challenges of other health sectors are worth considering. Telehealth is used in Australia in both an acute emergency (eg stroke treatment63) and for more routine consultations. A systematic review of Australian telehealth programs concluded that the key factors for success were vision, ownership, adaptability, economics, efficiency and equipment42. The authors concluded there is potential to scale up and replicate successful services in rural and remote settings42. A successful telehealth program for AMS has been described at the Goulburn Valley Health Service in Victoria46.
The review undertaken was not systematic and the selection of articles included may have, therefore, been subject to author bias. The description of hospitals in articles and the internet was not always sufficient to determine definitively whether the hospitals were in regional, rural or remote locales (eg for hospitals described as ‘community’ in the USA). However, the purpose of this review was to showcase models for AMS that overcame barriers in the regional/rural/remote setting, rather than be exhaustive in its scope or too limited in its examples.
It was not possible to provide a comparison of the effectiveness of the models presented because different measures were used to assess the programs. Few studies report patient outcomes or changes in resistance. Proxy markers such as antimicrobial usage, recommendation acceptance rates and cost savings were most often reported and therefore included in this overview.
There is a great opportunity for Australian regional, rural and remote hospitals to learn from each other’s experience with AMS program delivery (Fig3). Sharing key enablers in this setting is critical, and support for smaller sites to publish such information is necessary. By sharing such experiences, development of AMS programs in regional, rural and remote hospitals will be enhanced and the appropriate use of antimicrobials better supported.
 Figure 3: Take-home messages for regional, rural and remote hospitals.
Figure 3: Take-home messages for regional, rural and remote hospitals.
Conclusion
Regional, rural and remote hospitals require greater support to deliver comparable health care to metropolitan centres. This review highlights that AMS programs can be implemented in the regional, rural and remote setting, despite known barriers.
Australian regional, rural and remote hospitals can learn from each other’s experience with AMS program delivery, but this is limited by the lack of published information on these programs. Addressing this gap will assist regional, rural and remote hospitals to further promote optimal antimicrobial use within their facilities and further contribute to the minimisation of antimicrobial resistance.
Acknowledgements
The authors thank Arjun Rajkhowa (National Centre for Antimicrobial Stewardship) for editorial support. Jaclyn L. Bishop (nee Baker) is funded through an Australian Government Research Training Program Scholarship. The National Centre for Antimicrobial Stewardship is supported by the NHMRC Centre for Research Excellence scheme.
Conflict of interest
David CM Kong has sat on advisory boards for Merck, Sharpe & Dohme (MSD), and received financial/travel support unrelated to the current work from Roche and MSD.

