Introduction
Respiratory syncytial virus (RSV) is a leading cause of acute lower respiratory infections (ALRIs) in infants and young children (aged <5 years) and in older people, accounting for a high burden of morbidity and mortality1. In infants and young children, RSV-related ALRIs in young children are primarily bronchiolitis and pneumonia2,3. Globally, the impact of RSV is large, accounting for 33 million ALRI episodes, 3.2 million hospitalisations and 59 600 in-hospital deaths in those aged less than 5 years4. Nevertheless, these data likely underestimate the true disease burden as they do not account for RSV ALRIs in older children5, diagnostic misclassification from hospital-based coding practices6, and out-of-hospital RSV-attributable deaths in low- and middle-income countries4,7. To reduce RSV disease in young children, several candidate vaccines are currently undergoing development and clinical trials8,9. In preparation for introducing RSV vaccines, better epidemiological data on RSV are needed, including clinic attendance for ALRIs and data relevant to high-risk populations, such as Indigenous children3,9.
In Australia, Indigenous children have a high ALRI burden10,11, with hospitalisation rates as high as 427 per 1000 in Indigenous infants from the Northern Territory (NT)12. Indigenous children, especially from remote communities, are seven times more likely to be hospitalised with pneumonia than non-Indigenous children13. Moreover, hospitalised and recurrent ALRIs in the first few years of life are associated with an increased risk of future impaired lung health10, reduced lung function14,15 and bronchiectasis16-18. Indeed, the authors’ previous work among young children aged less than 24 months hospitalised with bronchiolitis showed that Indigenous children have more severe disease (eg longer duration of hospitalisation and supplemental oxygen requirement)19,20 and poorer clinical outcomes post-hospitalisation (re-hospitalisation for respiratory illnesses within 6 months) than non-Indigenous children19,20. Moreover, Indigenous children with prolonged cough 3–4 weeks after hospitalisation for bronchiolitis were at increased risk of future bronchiectasis (odds ratio (OR) 3.0, 95% confidence interval (CI) 1.1–7.0, p=0.03)21.
RSV is the commonest cause of hospitalised ALRIs in Australian children22. National as well as population-based data-linkage studies from the states of Western Australia and New South Wales found RSV hospitalisation incidence rates approximately two-to-four times higher in Indigenous than non-Indigenous children6,13,23. Similar disparities in RSV-related ALRIs between Indigenous and non-Indigenous children are reported in hospital-based studies from central Australia24 and the top end of the NT25. However, while community-based studies of RSV–ALRI are needed to capture the full spectrum of severity and for cost-effective analyses of future vaccines, few exist in Australia26-28.
In addition to the limited community-based data on RSV prevalence among Indigenous children in the northern Australian setting, data relating to co-detection of other viruses with RSV in this population are also scarce. Nevertheless, the clinical relevance of single versus multiple virus detections in ALRIs remains unclear, especially as 23–55% of virus detection in community-based surveillance studies are unaccompanied by any symptoms26,29-31.
To begin addressing this knowledge gap, the authors of this study determined the point prevalence of RSV in northern Australian children by conducting a secondary analysis of 11 hospital and community-based studies of children with ALRIs (principally bronchiolitis), otitis media and chronic pulmonary disorders, which included investigations during periods without acute respiratory illness and involved mainly Indigenous participants17,20,32-36. The secondary aim was to describe the prevalence of RSV co-detections with other respiratory viruses.
Methods
Study design and settings
Data were sourced from 11 prospective studies (Table 1). Eight involved children with either acute or chronic respiratory illnesses17,20,32-35, one involved children who acted as case-controls in study 4 and the remaining two focused on otitis media36. These were conducted in the NT, central Australia, and in the northern Queensland city of Townsville between 1996 and 2017 inclusive. One of the studies is ongoing, and the main results (reporting of primary aims) of seven studies and a substudy have been published already17,20,32-37.
Hospital-based studies included the Royal Darwin Hospital and Alice Springs Hospital in the NT, and the Townsville Hospital in Queensland. The Royal Darwin38, Alice Springs39 and Townsville hospitals40 are 360-, 186- and 580-bed teaching hospitals respectively with each containing the sole specialist paediatric services for their regions (Fig1). Community-based studies were undertaken across the NT, including central Australia, and in Queensland (communities vary according to the original studies, Table 1).
For each study, data custodians provided written approval for data to be accessed. Each original study received ethics approval with informed consent obtained for all nasopharyngeal swabs (NPSs) opting in to approve future analyses.
Table 1: Description of studies, clinical state and setting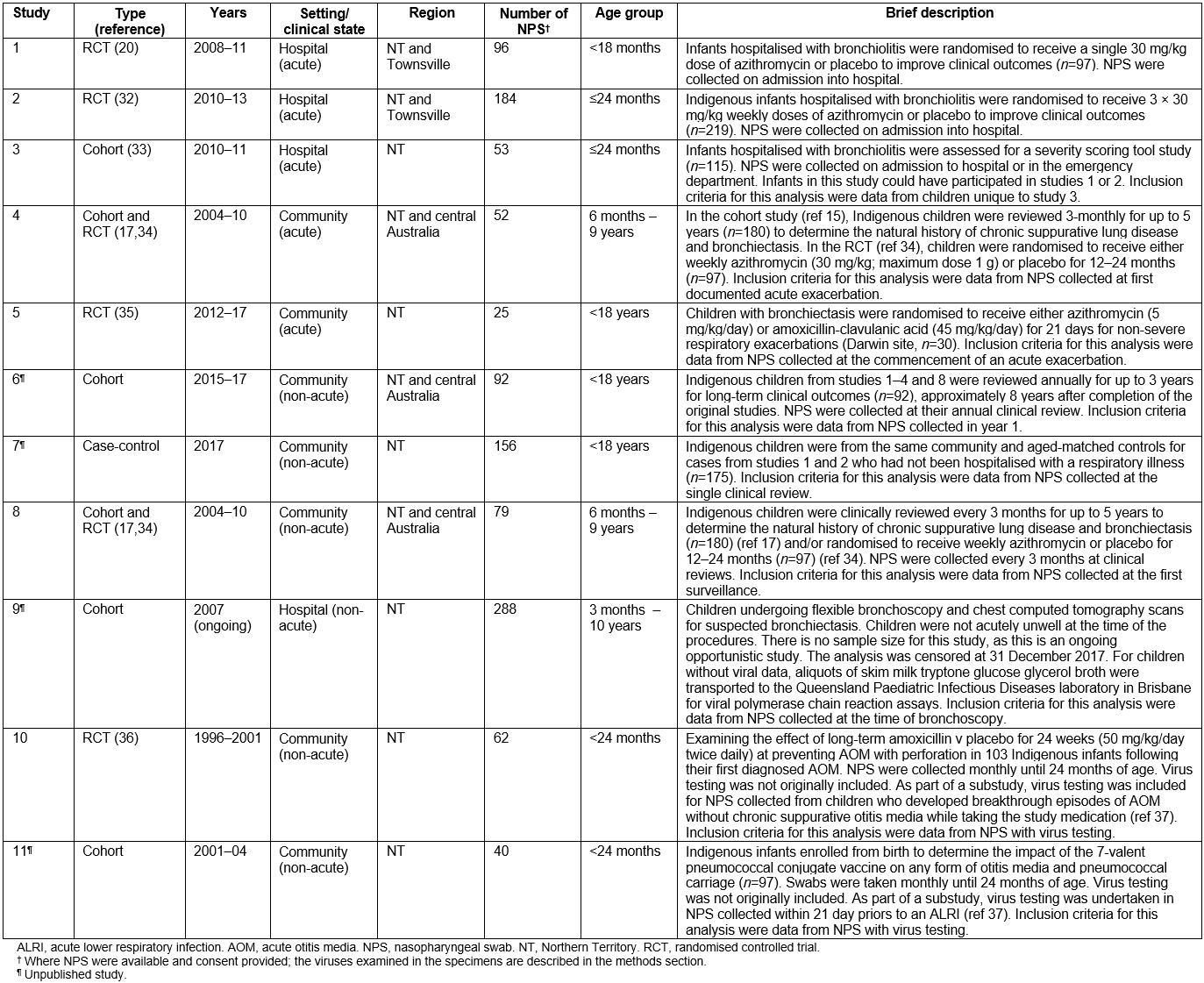
 Figure 1: Hospital catchment areas.
Figure 1: Hospital catchment areas.
Geographical region and climate
The NT consists of two main regions: the tropical north and central Australia. The tropical north has two distinct seasons: the dry (May to October) and wet (November to April) seasons. Central Australia (including the Anangu Pitjantjatjara Yankunytjatjara Lands, of northern South Australia) is a semi-desert region that has a typical four-season pattern. Townsville is a coastal city situated in north-eastern Queensland, with a tropical climate similar to the NT tropical north.
Study population
Children aged less than 18 years with recorded NPS virus results, or stored NPS specimens available, and consented for future testing, were eligible for inclusion. No other inclusion criteria were used, as eligibility criteria varied between each of the original studies (Supplementary table 1).
Supplementary table 1: Summary of included studies
Clinical data
A hierarchical approach was used to identify data from the original studies. For acute hospital or community-based studies17,20,32-35 where more than one clinical visit was recorded and children were either hospitalised or treated at the local health centre, ALRIs were defined according to previous clinical trials17,20,32-35. This was age-adjusted tachypnoea with wheeze or crackles in otherwise previously well infants and young children with bronchiolitis20,32,33, and any combination of increased cough, dyspnoea, increased sputum volume or purulence, haemoptysis, or new chest examination or radiographic findings in children with known chronic suppurative lung disease or bronchiectasis17,34,35. For non-acute studies17,34, the authors selected the first available healthy visit (ie no acute respiratory illness) with available virus testing data. For the otitis media-related studies36, the authors selected the first clinical visit where virus testing results were available.
Data custodians extracted de-identified data using a pre-specified list of variables (as available), including age, ethnicity, birth history, breastfeeding, family history, exposure to tobacco (during pregnancy and in the household), chronic pulmonary disorders, other co-existing illnesses (eg pyoderma, otitis media, rheumatic heart disease, faltering growth), setting (hospital/community), viruses, season (dry/wet season in the tropics of the NT and Queensland) and region (urban/remote). As per the authors’ previous studies, remoteness was defined as more than 100 km from a tertiary hospital20,32,33,41. Before merging data sets, variables were recoded to ensure identical coding between studies.
Virus data
In all studies, an NPS was collected and placed into skim milk tryptone glucose glycerol broth (STGGB), and stored at –80°C using standardised procedures at the Menzies School of Health Research42. Where virus result data were not already available, an aliquot of the stored STGGB was submitted by courier to the Queensland Paediatric Infectious Diseases laboratory in Brisbane, which had acted as the reference laboratory for all studies. The polymerase chain reaction assays used have been reported previously20,32,35 and included testing for RSV (A and B), HRV, human adenovirus, parainfluenza (1, 2, 3), influenza virus (A and B), human metapneumovirus, human enterovirus, human coronaviruses (NL63, OC43, 229E, HKU1), human bocavirus and human polyomaviruses (KI, WU).
Statistical analysis
Descriptive analyses were conducted using Stata v14 (StataCorp; http://stata.com). As data were not normally distributed, non-parametric measures were used. Patient characteristics are presented as numbers and percentages, median and interquartile ranges (IQR: 25th – 75th percentile). As this was an opportunistic study of available data, sample size calculation was not undertaken.
Ethics approval
The Human Research Ethics Committee of the NT Department of Health and Menzies School of Health Research approved the current study (HREC-17-3025).
Results
The demographic and clinical data for the 1127 children included in this study are summarised in Table 2. More children were from hospital studies (n=621) than community-based studies (n=506). The median age of children was 1.8 years (IQR 0.5–4.9); 58% were males, 90% were Indigenous, of whom 81% were from remote communities, and 57% had at least one co-existing illness. Exposure to tobacco smoke varied between studies and settings; however overall, it was very high during pregnancy (54%) and at home (73%). Overall, 77% of NPS collected from children during ‘well child’ (ie non-acute respiratory) visits and otitis media studies were conducted during the dry season, due to lack of accessibility to remote communities at other times of the year.
Table 2: Baseline characteristics by setting and condition 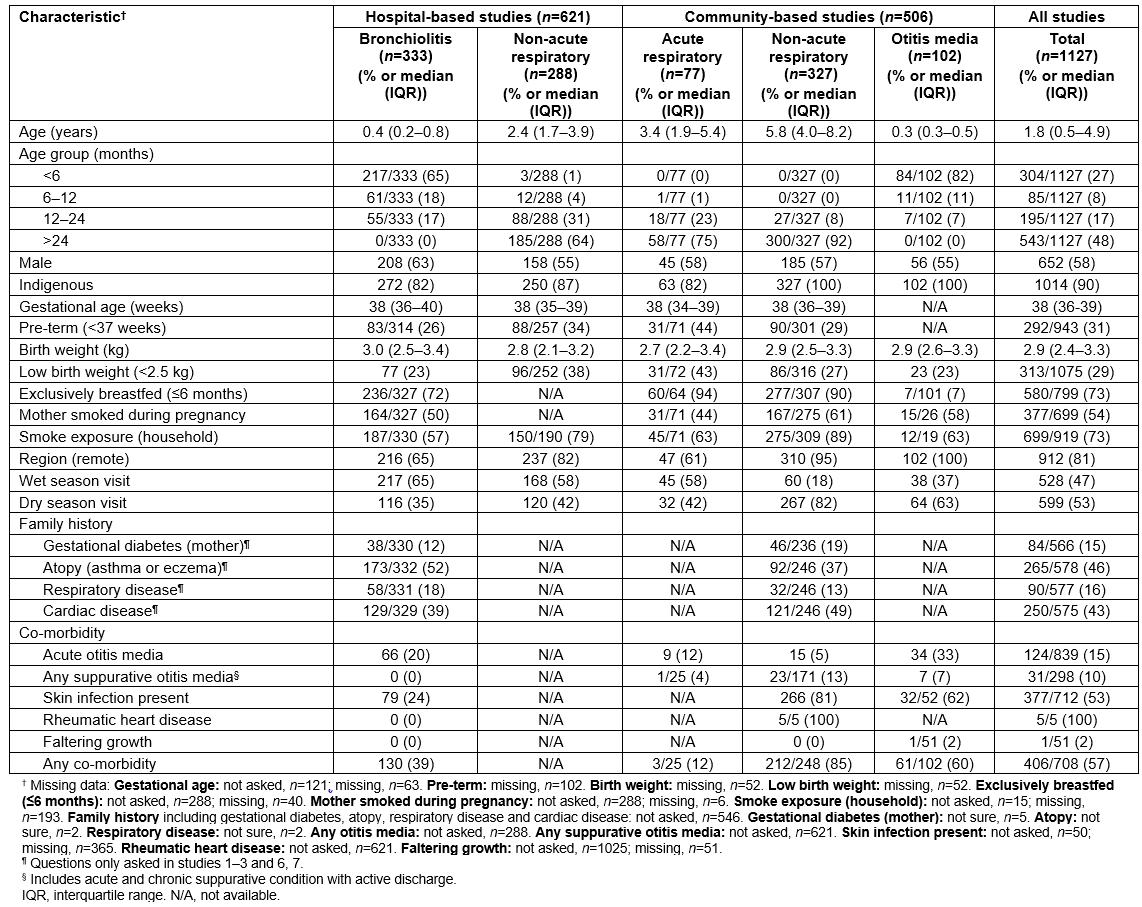
Prevalence of respiratory syncytial virus by setting, study type and condition
The overall point prevalence of RSV was 173/1127 or 15% (95%CI 13–18). RSV grouped by setting and condition are summarised in Table 3. The point prevalence of RSV was highest in hospital-based studies of bronchiolitis in children aged less than 24 months (156/333; 47%, 95%CI 41–52) and of these 107/156 (69%) were infants aged less than 6 months (95%CI 61–76). In contrast, RSV was detected in only 2.1% (17/794, 95%CI 1.3–3.4) of children across other clinical settings. This included children who were clinically stable and undergoing an elective bronchoscopy for chronic respiratory symptoms (study 9). In community-based studies of otitis media (studies 10 and 11), those undergoing a ‘well child’ outpatient review after hospitalisation for bronchiolitis or who were part of the case-control study (studies 6 and 7), and children with non-severe (non-hospitalised), acute pulmonary exacerbations of chronic suppurative lung disease or bronchiectasis (studies 4, 5 and 8), the prevalence of RSV was 1.4% (7/506, 95%CI 0.6–2.8).
Table 3: Prevalence of respiratory syncytial virus by setting and condition 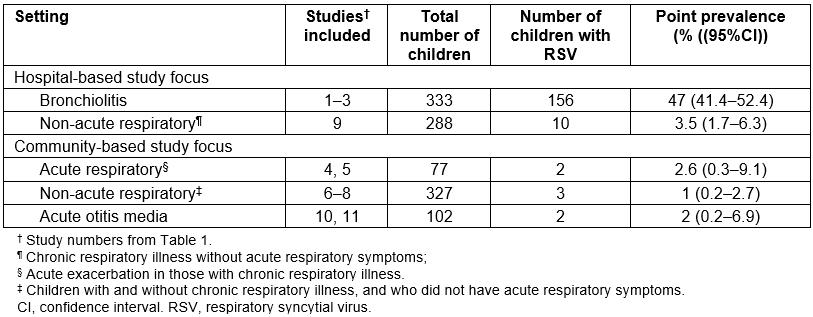
Other viruses, including RSV co-detections
Detection of other respiratory viruses in NPS samples varied according to setting and type of illness (Table 4). HRV was the virus detected most commonly in community-based studies, with highest detections in otitis media (prevalence 57%, 95%CI 47–67). The prevalence range for other viruses was 0–9%. At least one virus was detected in 706 (63%, 95%CI 59–68) children with 227 (20%, 95%CI 18–23) having two or more viruses present. Of the 173 RSV detections, 56 (33%, 95%CI 26–40) involved co-detection with other viruses and where HRV co-detection was the most common (27/173, 16%).
Table 4: Distribution of respiratory viruses and atypical bacterial pathogens by setting and condition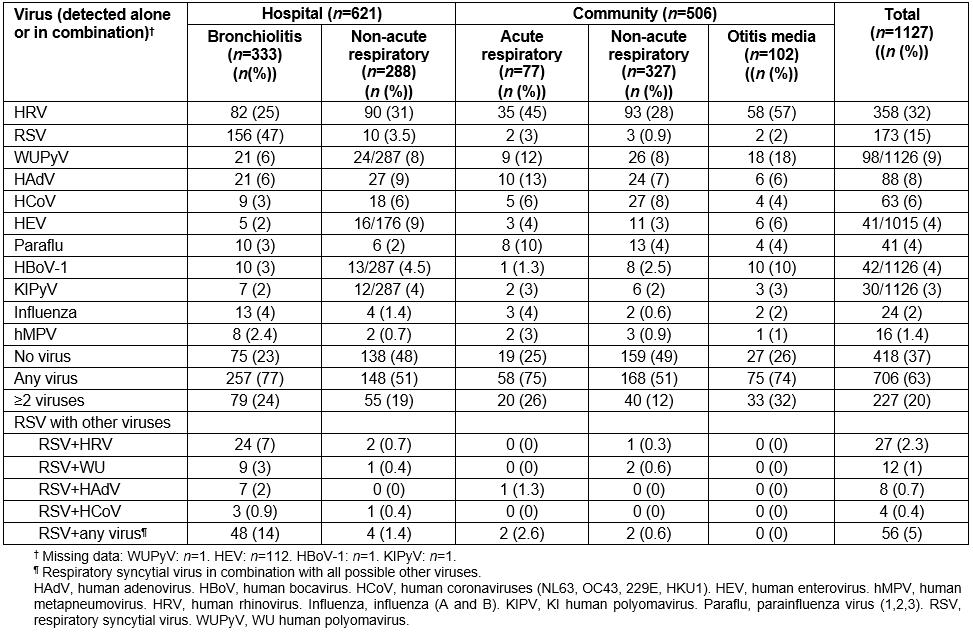
Discussion
This study provides RSV prevalence data for northern Australia derived from 11 prospective hospital- and community-based studies involving 1127 subjects in a setting where children are at high risk of acute and chronic respiratory illnesses2,10,11. While limited, it begins to address recommendations made by WHO to gather more information on local RSV epidemiology and disease burden in disadvantaged populations while awaiting future licensed RSV vaccines43. It was found that RSV was second only to HRV with an overall prevalence of 15% in the study region. RSV point prevalence of 47% was highest in children aged less than 24 months hospitalised with bronchiolitis, whereas prevalence in community-based studies was only 1.4% in children with either otitis media or in older children aged up to 18 years with or without ALRI respiratory symptoms. In contrast, HRV was common across all clinical settings and ages, including having a point prevalence of 30% in clinically stable children without either ALRI symptoms or otitis media. Finding HRV in asymptomatic children is not surprising as reported in several other studies, such as that described by Advani et al, where HRV was detected in near-equal numbers in hospitalised children with respiratory symptoms (41/148, 27.7%) and those without (34/158, 21.5%)29. Viruses other than RSV and HRV were uncommon, with point prevalence rates less than 10%. Co-detection of RSV with other respiratory viruses was 33% across all clinical settings.
The present study data are consistent with other hospital-based ALRI studies that also include Indigenous infants with bronchiolitis, where RSV was detected in 41–83% of patients4,44,45. In the present study, 31% of children hospitalised with RSV-related bronchiolitis were aged 6–24 months, when maternal RSV vaccination may no longer be protective,46, emphasising the importance of also developing active immunisation programs for young infants. In contrast to hospital-based studies, detection of RSV was low in the seven community-based studies (1.4% of children overall; 2.2% in those with and 0.9% without an acute respiratory illness).
It was not possible to calculate the population-based incidence of RSV-ALRI in our studies. In the Australian state of New South Wales, a data-linkage study determined RSV-hospitalisation incidence at 4.9 (11.0 in Indigenous children) per 1000 child years in those aged less than 5 years (peak incidence at age 0–3 months was 25.6 per 100 child years (58.0 in Indigenous children)23. Differences in the incidence of RSV is likely multi-factorial, and influenced by age, disease states, timing (season) and duration of surveillance4. Without active surveillance in the study region, the true incidence and disease burden of RSV are unknown, although a higher incidence of RSV-related ALRI in community studies is expected, due to the high burden of respiratory disease among Indigenous people compared to non-Indigenous people10,11. The most likely reasons for lower-than-expected rates of RSV detection in community studies include sampling from comparatively older children, and lack of acute respiratory symptoms26,27. Most of these children were seen in the dry season (May to October), rather than during the peak respiratory period in the wet season (November to April)25,47, and this is also likely be a contributing factor to differences in RSV detection.
Co-detection of other viruses with RSV was relatively common, occurring in one-third of cases. The most common co-detected virus was HRV, followed by the DNA viruses, human polyomavirus WU and adenovirus. The clinical relevance of virus co-detections in children with an ALRI remains uncertain31. In a recent community-based birth cohort study of young Australian children, single infections with RSV and human metapneumovirus were found to be most strongly associated with symptomatic ALRI; this study observed that new virus co-detections were also associated with a significantly increased attributed risk of symptoms26.
The present study has several limitations. No prospective longitudinal studies of RSV in at-risk Indigenous children have been reported in the study region. An Australian urban community-based study of healthy infants followed prospectively from birth to age 2 years28 observed RSV infections were uncommon until after age 6 months, following which there was a steady increase in RSV detections. By age 2 years, 58% of the cohort had at least one documented RSV infection. Similar findings were reported in a birth cohort from semi-rural villages in Vietnam48 and in Kenya49. Nevertheless, these studies may not represent children in the study region who are at high risk of severe ALRIs. This was an opportunistic secondary analysis of data from studies not designed specifically for determining RSV epidemiology. The data had limited seasonal coverage, thus not fully capturing annual outbreaks of RSV. Ninety percent of children were Indigenous, 81% of whom were from remote communities. A prospective, community-based study of non-Indigenous children in the region would be informative. A more comprehensive study is indicated to more accurately identify risk factors for RSV infection and priority target groups for future RSV vaccination. Lastly, the authors were unable to report mortality as these data were not collected.
Conclusion
This study combines hospital- and community-based studies of children aged less than 18 years who live in settings with a high burden of acute and chronic respiratory illness. The authors found RSV was second only to HRV as the most prevalent virus detected across all settings. RSV was the most frequently detected virus in infants hospitalised with bronchiolitis, including those aged less than 6 months. In contrast, RSV was uncommonly detected in children in community settings. While other respiratory viruses were often co-detected with RSV, their contribution to the child’s clinical state is uncertain. Ongoing surveillance of RSV50 in hospital and community settings is needed to further understand its epidemiology, particularly in at-risk populations (ie Indigenous) within Australia to guide prevention strategies and future RSV vaccine schedules. The young age of most RSV-positive hospitalised cases suggests a potential benefit from future maternal RSV vaccine programs.
Acknowledgements
We are grateful for the children and families who participated in the original studies. We thank Kim Hare and Jemima Beissbarth for their help in providing de-identified data.
References
Supplementary material is available on the live site https://www.rrh.org.au/journal/article/5267/#supplementary
You might also be interested in:
2012 - Prevalence of low back pain among peasant farmers in a rural community in South South Nigeria