Introduction
Diabetes mellitus (DM) is a metabolic disorder characterized by chronic hyperglycemia, resulting from defects in insulin secretion, insulin action or both1,2. The disease is associated with significant increased risk of long-term microvascular and macrovascular complications2. Currently DM has reached epidemic proportions, affecting more than 170 million individuals worldwide, with an estimated increase of at least 50% by 2010, especially in developing countries. In observational3 and intervention studies4,5, obesity and physical inactivity represent the most important modifiable risk factors for DM. Indeed, in subjects with pre-diabetes4,5, lifestyle intervention significantly and cost-effectively reduced the incidence of DM, thus justifying the implementation of population based strategies for identifying and treating high-risk individuals.
In the last decade there have been no retrospective studies with the object of assessing the frequency of DM in an agricultural population. At the same time there has been a large change in the living conditions and social status of that population.
The prevalence of DM varies widely among populations, due to differences in environmental influences and genetic susceptibility6. It is generally accepted that urbanization adversely affects prevalence, with rates significantly higher in urban than in rural communities7. In Greece, a limited number of population-based studies have been conducted regarding diabetes prevalence in urban and rural8-13 populations. Such studies used varying methodologies and are now relatively old. The objective of the present study was to determine the prevalence of diabetes and impaired glucose tolerance and their association with risk factors in the villages Saint Demetreus, Adami and Metohi of the district of Argolida, a representative rural area of Greece.
Methods
The study was conducted from March 2002 to October 2002, by six investigators. The study population included the resident population of the villages Saint Demetreus, Adami and Metohi of the district of Argolida according to the 2001 Greek Census. All subjects gave informed consent, for example:
The present study detects the prevalence of diabetes mellitus in the agricultural population of three indicative villages (Agios Dimitrios, Adami, Metohi). Your participation means that: you will answer the questions given to you, there will be blood and urine collection for our study. If further investigation is needed you will come to our Health Center for a new blood collection. If you refuse to participate, please inform us directly.
The study was approved by Ethics Committee of Tzanio Hospital, Greece. According to the demographic registration of 2001, the total village population was 1100. The study population consisted of all residents present during the 6 month period of registration. The village population is representative of the agricultural population because: (i) these villages are located in recognised traditional Greek agricultural regions; and (ii) the villages are located 200 m, 600 m and 900 m above sea level. All agricultural regions in Greece are thus located.
The entire population was screened by the 'door-to-door' method. As arranged in advance by civil authorities, the investigator visited each subject at home. After receiving the subject's consent, personal and family history was recorded. Subsequently subjects were invited to the health station after an overnight fast. Weight, height, and waist and hip circumference were measured and the body mass index (BMI) and waist to hip ratio (WHR) were determined. Subjects with BMI ≥ 30 kg/m2 were classified as obese and those with BMI ≥ 25 Kg/m2 as overweight. Central obesity was defined as WHR >0.85 in women and >0.90 in men. Systolic and diastolic blood pressure (sBP and dBP, respectively) was measured twice with the subject in the sitting position and after 15 min rest, and the mean of the two measurements was used. Arterial hypertension was defined as sBP ≥ 140 mmHg and/or dBP ≥ 90 mmHg, or the use of antihypertensive medications.
Blood samples were drawn for the determination of plasma glucose, total cholesterol, triglycerides and HDL-cholesterol using enzymatic methods. LDL-cholesterol was estimated by the Friedewald equation. In the case of fasting plasma glucose (FPG) >126 mg/dL, a second determination was performed one week later. Subjects with FPG <126 mg/dL on repeated test and those having FPG between 110 and 126 mg/dL were invited for a standard oral glucose tolerance test (OGTT). DM was defined according to the WHO criteria1. In addition, subjects with previous history of diabetes or who were taking oral hypoglycemic agents or insulin were considered to have DM. Subjects with known diabetes were not tested for FPG.
Statistical analysis
Data are presented as means (95% confidence interval). Normality was tested with the Kolmogorov-Smirnov test. Student's t-test was used for the analysis of normally distributed data and Mann-Whitney U-test for skewed data. Categorical variables were analyzed with Pearson's c2 test. Logistic regression models were built and tested with the 'Enter' method concerning the association of independent risk factors with DM. Variables with p<0.10 in univariate analysis were entered in multivariate analysis. In all analyses p<0.05 was considered statistically significant. Data analysis was performed using SPSS vers. 10.0 (SPSS Inc; Chicago, IL, USA).
Results
Data concerning the prevalence of diabetes according to age and sex are presented (Table 1). The total prevalence of diabetes was 7.4% (95%CI: 6.0-9.5), not significantly different between sexes (7.1% vs 7.6%, p = 0.61, for males vs females, respectively). Also, 5.2% of the total population had known diabetes, while eleven subjects (1.2%) had previously undiagnosed diabetes based on two consecutive FPG measurements. Furthermore eight subjects (0.9%) were identified as diabetic by OGTT. The prevalence of combined impaired glucose tolerance/impaired fasting glucose (IGT/IFG) was 3.7% (33/880), while isolated IFG was detected in 1.9% (17/880).
Table 1: Prevalence of diabetes mellitus according to age and sex
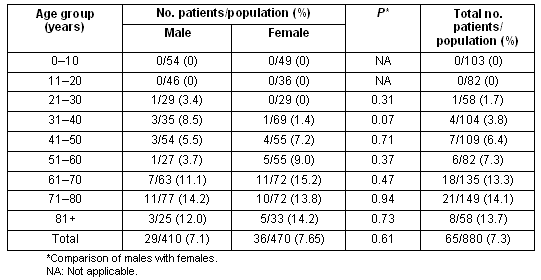
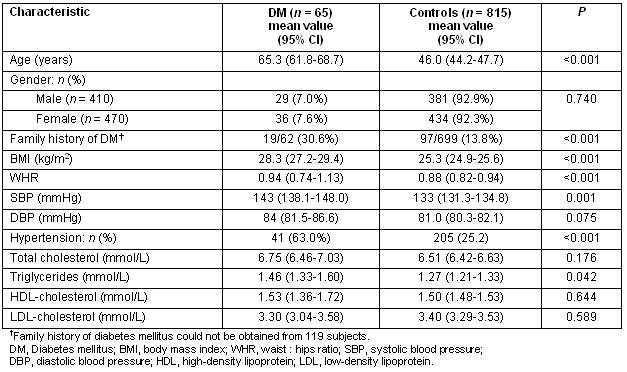
The prevalence of diabetes increased significantly with advancing age (Table 1). Indeed there was almost a doubling of the prevalence of diabetes after the age of 60 years (7.3 % vs 13.3%, for 51-60 years vs 61-70 years, respectively). The prevalence of obesity in the entire study population was 20.8%, significantly increased in patients with diabetes (36.0% vs 19.2, p<0.001, for diabetic vs controls subjects, respectively) (Table 2). Furthermore, central obesity was observed in 63% (95% CI: 59.4-66.6), again significantly more commonly in patients with diabetes (82.5% vs 58.1%, p<0.001, for diabetic vs controls subjects, respectively). Finally, arterial hypertension was detected in 28.4%, being more common in patients with diabetes (63.0% vs 25.2%, p<0.001, for diabetic vs controls subjects, respectively).
Table 2: Baseline characteristics of diabetic and non-diabetic subjects (controls)
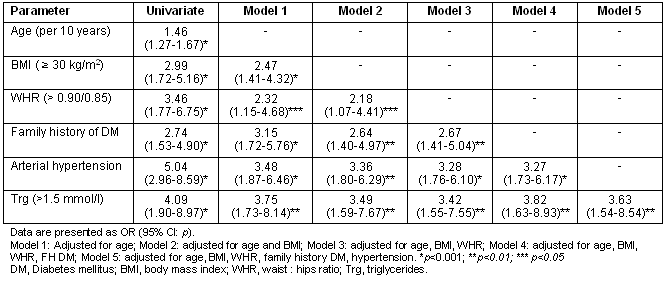
Regression analysis revealed that age (p<0.001), BMI (p<0.001), WHR (p<0.001, both as continuous and categorical variable), family history of diabetes (p<0.001), arterial hypertension (p<0.001) and fasting triglyceride levels (p<0.001) were significantly associated with the presence of DM (Table 3). Interestingly, stepwise regression analysis indicated that central obesity remained significant predictor of DM even after adjustment for BMI. Furthermore, family history of DM, arterial hypertension and fasting triglyceride levels remained significantly associated with DM even after adjustment for age and BMI/WHR.
Table 3: Univariate and multivariate regression analysis for the presence of diabetes mellitus
Discussion
The present study offers contemporary data regarding diabetes prevalence in a representative rural area of Greece. The total prevalence of diabetes was 7.3%, with no significant difference between genders, increasing significantly with age, while undiagnosed diabetes using FPG and the OGTT in patients with impaired fasting glycemia was 2.15%, indicating that approximately 30% of the total diabetic population had undiagnosed diabetes. Furthermore, impaired glucose regulation (isolated IFG and combined IFG/IGT) affected 5.6% of the population; accordingly, abnormal glucose tolerance was detected in 13% of the population studied.
Previous epidemiologic studies from Greece concerning diabetes prevalence were confined to urban populations8,9, have been retrospective11-13, used different diagnostic criteria10 or did not use the door-to-door method as a screening survey in the all population combined with FPG determination and OGTT8-13. Although the door-to-door method may miss approximately 11% of diabetes cases, it is more sensitive compared with GP registers14. Nevertheless, until 1993 rural studies reported age-adjusted prevalence of DM of 1.31%-1.36% in males and 1.48%-1.68% in females, while Lionis et al.11 in a recent retrospective case note review reported an age-adjusted known DM prevalence of 5.2%. Although this estimate is significantly higher compared with the older studies, it is similar to our results, where the prevalence of known DM was 5.2%.
It is firmly established that urbanization affects prevalence, with rates higher in urban areas than in rural communities7,15. A recent Greek study16 concerning metabolic syndrome in 4153 subjects in both urban and rural areas, reported an age- and sex-adjusted prevalence of DM of 10.6% (diagnosed by FPG or use of hypoglycemic medications), IFG of 7.6%, while undiagnosed diabetes was detected in 34% of the cases. Although the authors did not separately report the prevalence of DM in urban and rural areas, surprisingly, the frequency of metabolic syndrome was not different between areas. Nevertheless a recent epidemiologic study from Adana, Turkey17 did not observe a difference in diabetes prevalence between urban and rural areas, the findings reflecting changes in the frequency of obesity and lifestyle.
Several factors affect the prevalence of diabetes18, such as: (i) the ratio of diagnosed : undiagnosed cases of diabetes; (ii) age at onset of diabetes; (iii) population demographic changes; (iv) mortality in patients with diabetes and in the general population; and (v) incidence of diabetes. Indeed, in our study the number of undiagnosed cases is comparable with or lower than several other studies, indicating improved application of health-care services during the last decades in Greece. Furthermore, compared with older studies, there is a tendency of increased age-specific prevalence of DM particularly in younger subjects. Thus Katsilambros et al8 reported a prevalence of DM of 1.96%-0.93% (males-females, respectively) in the age group 40-49 years, while the corresponding numbers in our study were 5.5-7.2%.
It is well established that the aging of a population affects both the prevalence and the incidence of type 2 DM19,20. Beside the fact that age as a continuous variable was associated with a 45% increased risk for the presence of diabetes, there was almost a doubling of DM cases in subjects older than 60 years. In addition, obesity is a recognized factor associated with an increased risk for type 2 diabetes20,21. Indeed in our study, BMI, both as a continuous and a categorical (≥ 30 kg/m2 index of adiposity was associated with at least doubling the risk for the presence of DM. Furthermore, WHR, an index of central fat distribution, was significantly associated with prevalent DM, even after adjusting for BMI. These findings underlie the essential role of central fat distribution in the pathogenesis of type 2 DM22,23. Given the alarming increase in the prevalence of obesity24 and the favorable effect of lifestyle intervention in prediabetic subjects4,5 and in normoglycemic males at increased risk for future cardiovascular events25, there is an urgent need for the implementation of primary prevention strategies.
Several lines of evidence firmly establish that a family history of DM (FHDM) is a significant risk factor for prevalent and incident DM2,17,26. This increased risk is probably the combined result of genetic and environmental factors, affecting beta-cell insulin secretory capacity and peripheral insulin sensitivity. In our study, FHDM was associated with at least doubling the risk for prevalent DM. Although no data exist concerning the effect of primary prevention strategies in normoglycemic subjects with FHDM, it seems justified that this group should be a primary target for lifestyle intervention. Finally, arterial hypertension and fasting triglyceride levels were significantly and independently associated with a 3.2 to 3.6 increased risk for the presence of DM. Thus it is reasonable that these patients, especially if they have other features of metabolic syndrome, such as obesity, should be screened for the presence impaired glucose regulation and treated appropriately.
The ratio of prevalence of glucose abnormalities between men and women has been estimated in many studies, but so far there has been no consistent trend7,26,27. Indeed, the age-specific prevalence of undiagnosed diabetes and IGT defined by isolated post-load hyperglycemia was higher in women, while undiagnosed diabetes and IFG detected by isolated fasting hyperglycemia was higher in men. Furthermore, the total prevalence of diabetes seems to be higher in men aged 40-59 years, but lower in those over 70 years13. Nevertheless, earlier studies in Greece reported a non-significant trend for increased age-adjusted prevalence of DM in females8,11,12. It is possible that differences concerning the reference population, the age distribution of the sample and the screening methods applied, account for the observed variation.
The findings of our study have several implications for rural practice that should lead to modification of both screening methods and medical interventions applied. Indeed the prevalence of diabetes mellitus and impaired glucose regulation in a representative rural area of Greece is increased, with a significant proportion still undiagnosed. Central obesity and arterial hypertension seem to be common metabolic disorders in our region. Given that age, hypertension, obesity, family history of diabetes and elevated triglyceride levels are significantly associated with prevalent diabetes, it is justified these subjects should be a primary target for preventive intervention strategies.
References
1. Anon. Definition, diagnosis and classification of diabetes mellitus and its complications, report of a WHO Consultation. Part 1: Diagnosis and classification of diabetes mellitus. Geneva: WHO, 1999; 1-65.
2. Stumvoll M, Goldstein BJ, van Haeften TW. Type 2 diabetes: principles of pathogenesis and therapy. Lancet 2005; 365: 1333-1346.
3. Hu FB, Manson JE, Stampfer MJ et al. Diet, lifestyle, and the risk of type 2 diabetes in women. New England Journal of Medicine 2001; 345: 790-797.
4. Knowler WC, Barret-Connor E, Fowler SE et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. New England Journal of Medicine 2002; 346: 393-403.
5. Tuomilehto J, Lindstrom J, Eriksson J et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. New England Journal of Medicine 2001; 344: 1343-1350.
6. Amot A, McCarty D, Zimmet P. The rising global burden of diabetes and its complications: estimates and projections to the year 2010. Diabetic Medicine 1997; 14(Suppl 5): S1-S85.
7. King H, Rewers M: Global estimates for prevalence of diabetes mellitus and impaired glucose tolerance in adults: the WHO AdHoc Diabetes Reporting Group. American Diabetes Care 1993; 16: 157-177.
8. Katsilambros N, Aliferis K, Darviri C et al. Evidence for an increase in the prevalence of known diabetes in a sample of an urban population in Greece. Diabetic Medicine 1993; 10: 87-90.
9. Katsilambros N, Steryotis J, Moiras N et al. Prevalence of diabetes among glycosuric individuals in an urban area of Greece. Acta Diabetologica Latina 1977; 14: 211-218.
10. Christakopoulos P, Karamanos B. The prevalence of diabetes mellitus: epidemiological studies in Greece. Medicographia 1987; 9(Suppl 1): 44-46.
11. Lionis C, Sasarolis S, Koutis A et al. Measuring the prevalence of diabetes mellitus in a Greek primary health care district. Family Practice 1996; 13: 18-21.
12. Lionis C, Bathianaki M, Antonakis N, Papavasiliou S, Philalithis A. A high prevalence of diabetes mellitus in a municipality of rural Crete, Greece. Diabetic Medicine 2001; 18: 768-769.
13. Papazoglou N, Mannes CH, Chatzimitrofanous P et al. Epidemiology of diabetes mellitus in the elderly in northern Greece. A population based study. Diabetic Medicine 1995; 12: 397-400.
14. Simmons D, Gatland B, Fleming C et al. Prevalence of known diabetes in a multiethnic community. New Zealand Medical Journal 1994; 107: 219-222.
15. Motala AA. Diabetes trends in Africa. Diabetes Metabolic Research Review 2002; 18: S14-S20.
16. Athyros VG, Bouloukos VI, Pehlivanidis AN et al. for the MetS-Greece Collaborative Group. The prevalence of the metabolic syndrome in Greece: The MetS-Greece Multicentre Study. Diabetes, Obesity and Metabolism 2005; 7: 397-405.
17. Gokcel A, Ozsahin AK, Sezgin N et al. High prevalence of diabetes in Adana, a Southern Province of Turkey. Diabetes Care 2003; 26: 3031-3034.
18. Colagiuri S, Borch-Johnsen K, Glümer C, Vistisen D. There really is an epidemic of type 2 diabetes. Diabetologia 2005; 48: 1459-1463.
19. DECODE study group. Age- and sex-specific prevalences of diabetes and impaired glucose regulation in 13 European cohorts. The DECODE study group. Diabetes Care 2003; 26: 61-69.
20. Bonora E, Kiechl S, Willeit J et al. Population-based incidence rates and risk factors for type 2 diabetes in white individuals. The Bruneck Study. Diabetes 2004; 53: 1782-1789.
21. Dunstan DW, Zimmet PZ, Welborn TA et al. The rising prevalence of diabetes and impaired glucose tolerance. The Australian Diabetes, Obesity and Lifestyle Study. Diabetes Care 2002; 25: 829-834.
22. Ohlson LO, Larsson B, Svardsudd K et al. The influence of body fat distribution on the incidence of diabetes mellitus. Diabetes 1985; 34: 1055-1058.
23. Lebovitz HE, Banerji MA. Point: visceral adiposity is causally related to insulin resistance. Diabetes Care 2005; 28: 2322-2325.
24. Mamalakis G, Kafatos A. Prevalence of obesity in Greece. Internal Journal of Obesity 1996; 20: 488-92.
25. Smith GD, Bracha Y, MS; Svendsen KH, for the Multiple Risk Factor Intervention Trial Research Group. Incidence of type 2 diabetes in the randomized multiple risk factor intervention trial. Annals of Internal Medicine 2005; 142: 313-322.
26. Glumer C, Jorgensen T, Borch-Johnsen K. Prevalences of diabetes and impaired glucose regulation in a Danish population. The Inter99 study. Diabetes Care 2003; 26: 2335-2340.
27. Harris MI, Flegal KM, Cowie CC, Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults. Diabetes Care 1998; 21: 518-524.

