Introduction
The increasing burden of kidney disease and type 2 diabetes mellitus (T2DM) is a worldwide problem, especially for remote and Indigenous populations1-3.
The average life expectancy at birth of Aboriginal Australians is significantly lower than for non-Aboriginal Australians (by at least 8 years) and the disparity is more marked in remote areas4,5. Contributing factors to this reduced life expectancy are the effects of T2DM, kidney disease, their associated conditions and complications4.
At the time of this study the estimated prevalence of T2DM in Australian Aboriginal people ranged from 8.2% to 20.8% and estimated prevalence of impaired glucose tolerance ranged from 4.7% to 21.1%4,6. Prevalence increases with age and remoteness for both Aboriginal and non-Aboriginal people3,7. Aboriginal women are reported to be at particular risk7. Obesity, T2DM, albuminuria, hypertension, nephritis, poverty and living in remote locations are associated with increased risk of kidney disease, particularly for Aboriginal people4,6,8.
Prior to this study T2DM had been found to be the leading cause of avoidable mortality for Aboriginal residents of the Goldfields region of Western Australia, accounting for 20% of deaths9. Diseases of the kidney accounted for almost 6% of avoidable mortality9. The Goldfields has been estimated to have the second highest rate of end-stage renal disease in Australia10. There has been very little exploration of the possible reasons for this disparity.
The primary aim of the Western Desert Kidney Health Project (WDKHP) was to determine the prevalence of T2DM, kidney disease and the risk factors for these diseases in Aboriginal adults and children in a remote area of Western Australia. This study also compared the prevalence for non-Aboriginal adults and children living in the same locations, and national rates.
Methods
This study was conducted in the Goldfields and Western Desert, which starts 500 km east of Perth and extends 2000 km to the border with South Australia. Kalgoorlie is the only sizable town (30 000 people) (Fig1). The study communities were small and in remote and very remote areas of Australia, with extremes of temperature, weather and facilities, so the practical challenges were great and the number of participants relatively small (Fig2).
The study included six remote Aboriginal homeland communities with populations between 15 and 200 people. In homeland communities access is restricted to Aboriginal people with cultural connections to the area and people providing services such as teachers, nurses and administration staff. The study also included five small outback towns with populations from 180 to about 900 people. The residents of these towns include Aboriginal and non-Aboriginal people and families who choose to live in the region as well as government workers, people who work at the surrounding mines, people who provide services to the mines and community and some residents who commute to larger centres for work.
The WDKHP featured whole-of-community cross-sectional surveys and health assessments using point-of-care (POC) testing. It was conducted in five towns and six remote Aboriginal communities over lands of people of Western Desert language groups – primarily Wongutha, Mulba-Ngadu and Anangu-Pitjantjatjara language groups. The major regional town, Kalgoorlie, was not included in the study as it is more than 10 times larger than even the largest town in this study, has a very different pattern of service provision and the methods used in this study would not have been applicable.
Participation was offered to all people regardless of age or ethnicity (Fig2).
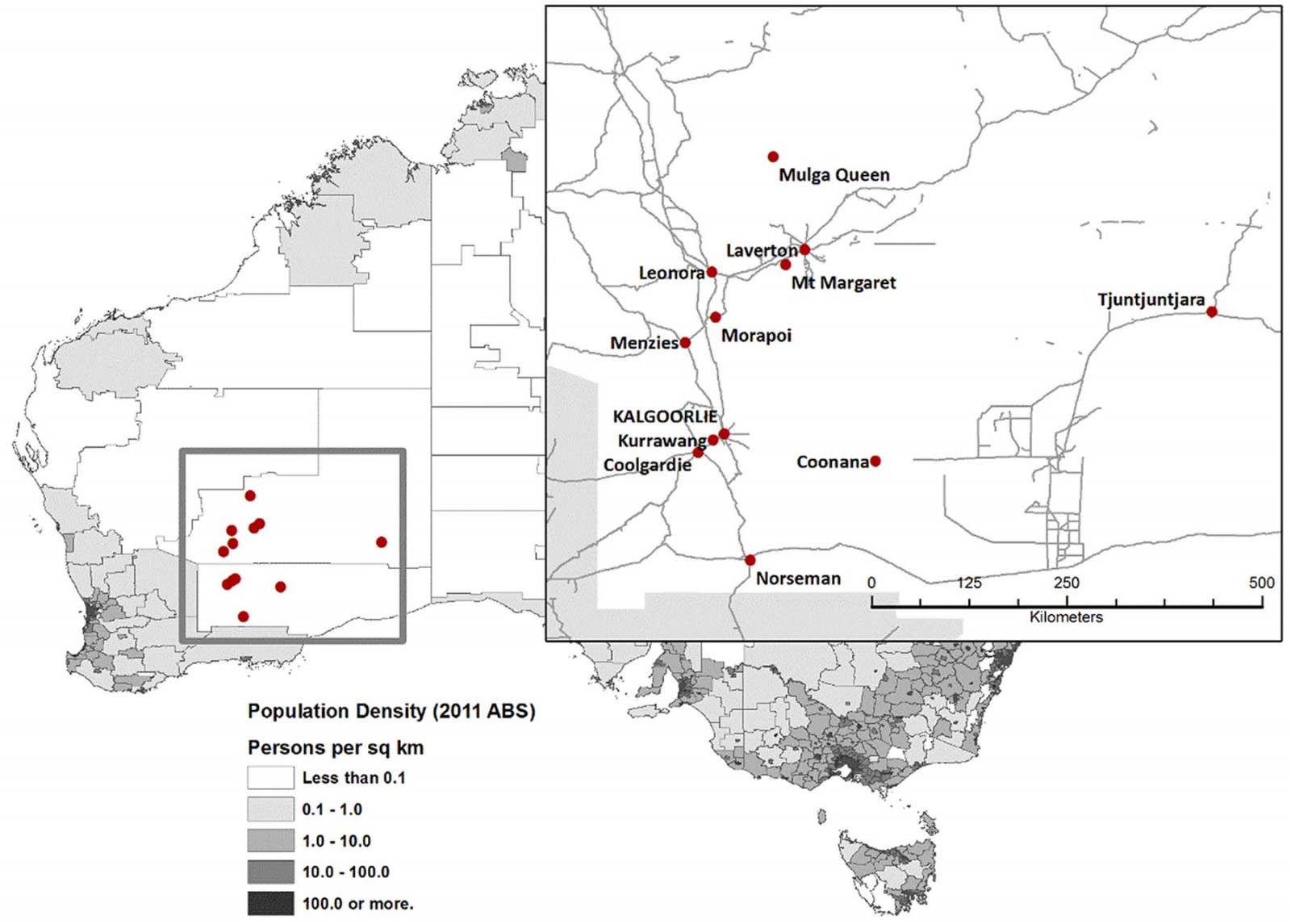 Figure 1: Map of the Western Desert Kidney Health Project study area.
Figure 1: Map of the Western Desert Kidney Health Project study area.
 Figure 2: Western Desert Kidney Health Project Data collection in a remote Western Australian town.
Figure 2: Western Desert Kidney Health Project Data collection in a remote Western Australian town.
Public involvement and consent
The team was led by an experienced Aboriginal researcher (AS) who has cultural seniority in the tribal groups of the region. Extensive consultation with, involvement of and communication with participants and their communities was a feature of project design and occurred at all stages of the project11. At enrolment participants and families were provided with verbal and written information about the study. Participants completed a written consent form or, where literacy prevented completion of the form, verbal consent was documented. Parents or guardians gave consent for children. The children were asked for assent and given the opportunity to withdraw from the study.
Data sharing
Several very small Indigenous communities are involved in this study. The communities and individuals could potentially be identified from the data, and the communities have been assured that their confidentiality and privacy would be protected as a condition of their consent to participate in the study. Application to the West Australian Aboriginal Health Ethics committee and the research team would be required for researchers who meet the criteria for access to confidential data to be given permission to access the data for this study.
Data collection
Starting in 2010 a mobile research team, including AS, Aboriginal health workers, doctors, collaborating researchers, artists and medical students, spent 2–3 weeks annually for 3 years in each of the study localities. The team travelled with a four-wheel-drive Clinic on Wheels and a support vehicle to transport equipment to support innovative community engagement strategies11.
There was good participation from Aboriginal people and others who came into direct contact with the study team. In the remote Aboriginal homeland communities everyone who was present during the testing participated in the study. In the remote towns participation was very good from people who were there during the day, especially for children attending school. People who were elsewhere for work were not available to participate in the study, and the numbers are difficult to estimate.
After consent was obtained, participants or their carers answered a questionnaire to record medical, family and dietary history followed by a clinical assessment and investigations using POC machines: Accutrend GC (Boehringer Mannheim) for blood sugar, DCA Vantage (Bayer) for ACR and glycated haemoglobin (HbA1c), and Clinitek Status (Bayer) for urinalysis. The Bega Garnbirringu Aboriginal Health Service Quality Assurance Team calibrated the POC machines after each community visit.
POC tests were performed immediately and samples disposed of in keeping with cultural beliefs that required no samples be taken away or kept. Participants did not have to undress for testing (also an important cultural requirement) – skin infection was assessed by self-report and examination of exposed areas. Acanthosis nigricans was used as a clinical indicator of hyperinsulinism and was assessed by examination of the axilla and back of the neck12.
Height and weight were measured with a personal weighing scale and stadiometer. Adults were categorised as underweight (body mass index (BMI) <18.5 kg/m2), normal (BMI 18.5–24.9 kg/m2), overweight (BMI 25.0–29.9 kg/m2) or obese (BMI >30 kg/m2). Age-specific values were used to classify children as underweight, normal, overweight or obese13. Participants with history, current treatment or HbA1c >6.5% on screening were considered to have T2DM14.
Three blood pressure measurements were taken using electronic blood pressure machines with appropriate size cuffs (Omron IA1B). After exclusion of obvious errors, measurements were converted to an estimated mean arterial pressure: MAP = ((2×diastolic) + systolic)/3). The minimal value for MAP of the three measurements was then identified to be the most appropriate blood pressure. Adult participants with history, existing diagnosis or confirmed current systolic ≥140mmHg systolic or ≥90mmHg diastolic were considered to have hypertension. Children found to have systolic and/or diastolic blood pressures above the 95th percentile for age were considered to be hypertensive15.
Normal albumin–creatinine ratio (ACR) for adults was <2.5 mg/mmol, elevated was considered to be 2.6–25 mg/mmol, and high was >25 mg/mmol. For children, ACR <3.5 mg/mmol was considered to be normal.
Data were recorded onto paper data sheets and later entered into a database constructed using EpiData v3.1(EpiData; http://www.epidata.dk) and exported to SAS v9.3(SAS Institute; http://www.sas.com). Participants were given immediate verbal and written feedback about their results. Those found to have abnormal results were referred to the doctor or health clinic of their choice for further investigation. Combined results were provided to the community at the end of each residency.
Statistical methods
Analyses for adults and children were carried out separately. Categorical factors were tested for associations with Aboriginality and gender using χ2 or Fisher’s exact test. Recategorised BMIs were compared using the Mantel–Haenszel test of association, and age was compared using the Kruskal–Wallis test because they were not normally distributed. Standardised morbidity ratios (SMRs) were calculated using the direct method adjusted for age separately for Aboriginal and non-Aboriginal participants for all variables where this information was available from national standards. Variables were included in the logistic regression for multivariate comparisons if the univariate association was p<0.2 and retained in the model if the adjusted significance was p<0.15. Variables not meeting these criteria are not included in the tables. All analyses were made using SAS.
Ethics approval
Ethics approval for this project was given by the Western Australian Aboriginal Health Ethics Committee (ref 287 05/10) and the University of Western Australia Human Research Ethics Committee (ref RA/4/1/4315).
Results
This article presents information from the initial health assessments for all participants (Tables 1–3). Data collection was more than 93% complete; however, at times extreme temperatures affected the POC machines, resulting in missing data for some denominators.
Using 2011 Australian Bureau of Statistics (ABS) estimates of the population in the study region, 79% (817/1035) of the Aboriginal population and 11% (282/2475) of the non-Aboriginal population completed at least one health assessment16. All non-Aboriginal people living in remote communities participated in the study (Table 1).
All Aboriginal adult participants were born in Western Australia; 95.8% had spent their childhood in the Goldfields. Of the non-Aboriginal adults 91.5% had grown up in an urban centre (p<0.001). At the time of participation 34.5% of Aboriginal and 9.8% of non-Aboriginal adult participants were living in remote communities. Most of the Aboriginal children in this study (95.9%) had spent their lives in rural or remote areas whereas 61.4% of non-Aboriginal children, although currently residing in a remote area, reported spending the majority of their lives in urban areas.
Table 1: Western Desert Kidney Health Project participant demographic characteristics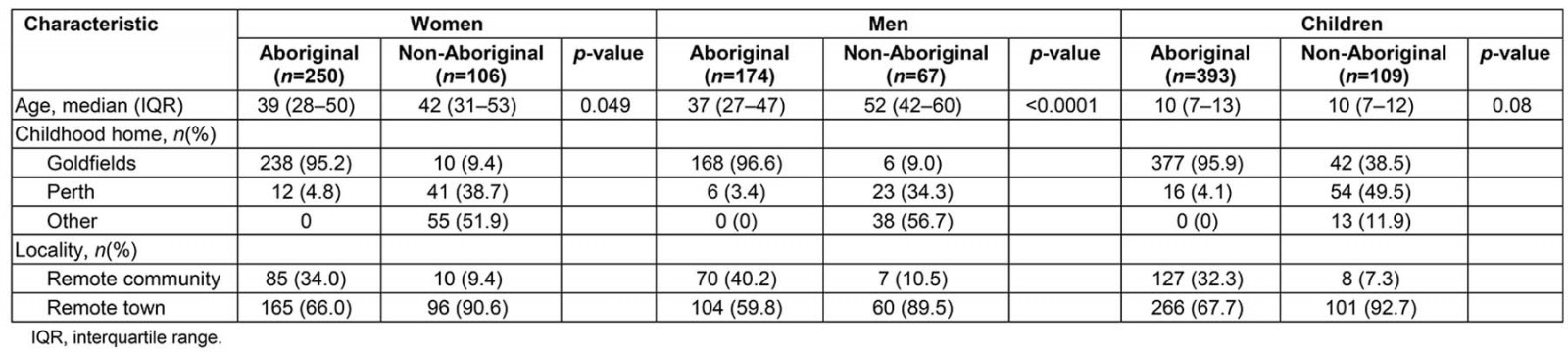
Age
Non-Aboriginal men and women taking part in the study were older than the Aboriginal participants. The age of the youngest participant was 8 months. The age distribution for Aboriginal and non-Aboriginal girls was similar although the Aboriginal boys were slightly older than the non-Aboriginal boys.
Biomedical markers
Biomedical markers are presented in Table 2.
Table 2: Clinical results for Western Desert Kidney Health Project initial health screen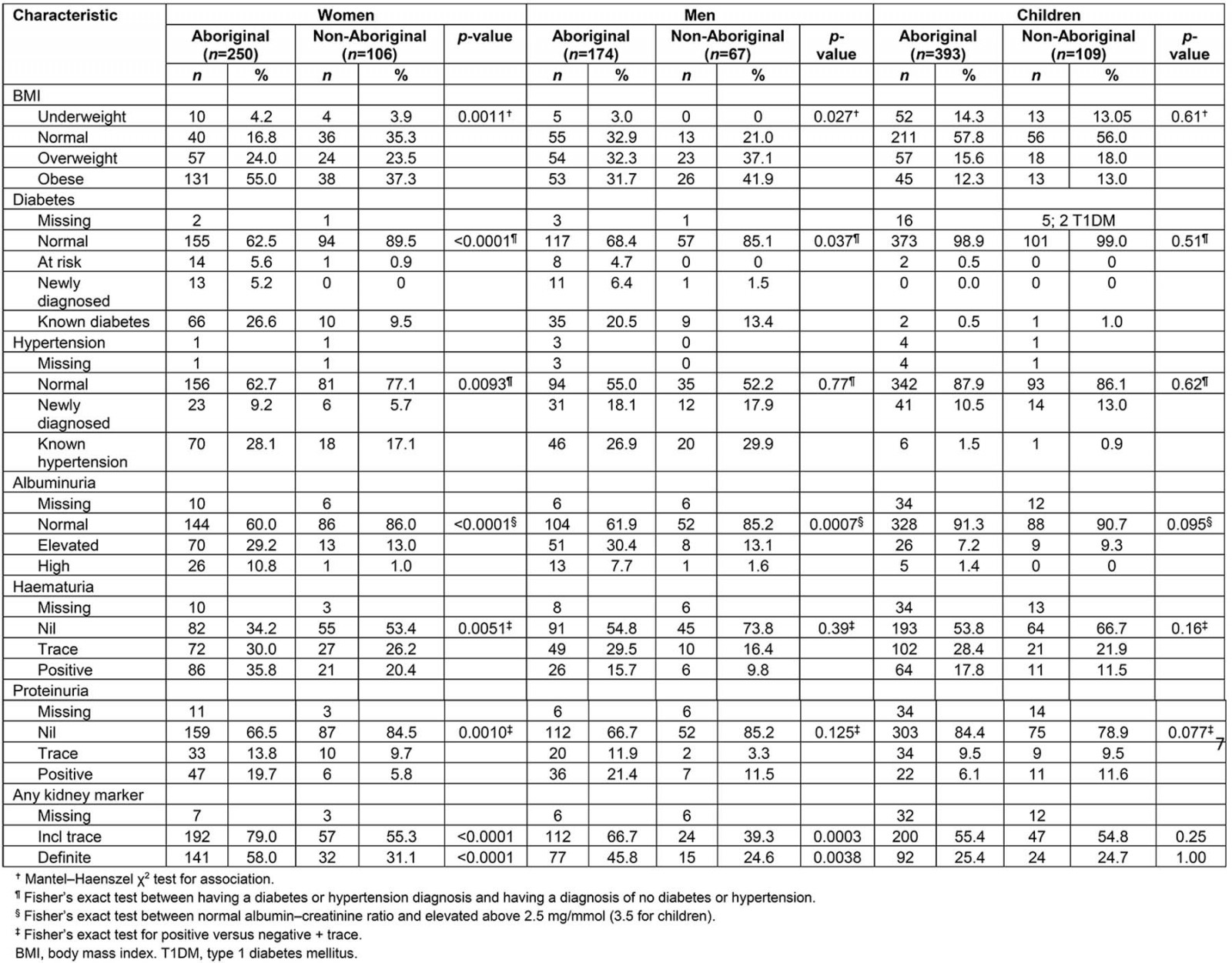
BMI
Being overweight or obese in the study group compared to the rate quoted by the ABS was not significantly different when adjusted for age (Aboriginal SMR 1.03, 95% confidence interval (CI) 0.92–1.16; non-Aboriginal SMR 1.01, 95%CI 0.83–1.21)17. For women BMI was significantly associated with Aboriginality (p=0.002) and age (p=0.021) but not with parity (p=0.72).
Rates of being overweight and obese were similar for Aboriginal and non-Aboriginal children and also similar to national figures. Being obese or overweight was apparent by the age of 5 years for some children.
Diabetes
The WDKHP identified 24 Aboriginal adults and one non-Aboriginal adult with a new diagnosis of T2DM (HbA1C>6.5%)14. Twenty-four percent of Aboriginal adults and 11% of non-Aboriginal adults reported they knew they had diabetes. Of these, 54 Aboriginal and seven non-Aboriginal people had T2DM that was poorly controlled (HbA1C ≥7%). Aboriginal and non-Aboriginal adults in the WDKHP had a higher burden of diabetes than the ABS standard population6 (Aboriginal SMR 2.23, 95%CI 1.86–2.66; non-Aboriginal SMR 2.00, 95%CI 1.22–3.09). Two non-Aboriginal children had type 1 diabetes; one non-Aboriginal and two Aboriginal child participants were known to have T2DM. The age of the youngest child with elevated HbA1c was 9 years.
Acanthosis nigricans was detected in 34.8% of Aboriginal women and 18.3% of Aboriginal men in the study. It was observed in 18.0% of Aboriginal girls and 10.2% of Aboriginal boys (p=0.032). Acanthosis nigricans was not seen in any children who were underweight. It was detected in 10% of children of normal weight, 23% of overweight children and 51% of obese children. The age of the youngest child with acanthosis nigricans was 2 years. It was detected in almost a quarter of children aged over 10 years. It was not recorded for non-Aboriginal participants as it is difficult to detect in people of Caucasian origin.
Infections
Non-Aboriginal women were more likely to report skin infection than Aboriginal women (20.8%, p=0.023) but men reported similar rates of infection (15% (non-Aboriginal men) and 21% (Aboriginal men), p=0.33). Skin infection was most common in non-Aboriginal boys (30.2%) – twice as frequent as Aboriginal boys (13.5%, p=0.020) and Aboriginal (12.5%) and non-Aboriginal girls (13.5%). Skin infection was reported in all age groups.
Hypertension
National figures predicted that between 19.5% and 23.0% of Aboriginal and non-Aboriginal adults would be hypertensive6,17. WDKHP rates (measured or on treatment) were 40.4% Aboriginal and 32.6% non-Aboriginal, with almost 20% of men and 8% of women not previously diagnosed found to be hypertensive. Hypertension rates were higher than the Australian population for Aboriginal women (SMR 1.90, 95%CI 1.54–2.31), Aboriginal men (SMR 1.83, 95%CI 1.46–2.28) and non-Aboriginal men (SMR 1.88, 95%CI 1.31–2.63) but not for non-Aboriginal women (SMR 1.26, 95%CI 0.83–1.85).
Sixty-two children (12%) were hypertensive, including six Aboriginal children and one non-Aboriginal child who were currently on treatment for hypertension. There was no difference in the rates of hypertension for Aboriginal and non-Aboriginal children.
Urine analysis
Albuminuria: Elevated ACR was present at all ages from 2 to 16 years in both groups of children. Aboriginal adults had albuminuria (ACR >2.5 mg/mmol) at a similar rate to national figures, but non-Aboriginal adults had a higher rate than predicted18,19. The age of the youngest child with elevated ACR was 2 years; 8.6% of Aboriginal and 9.3% of non-Aboriginal children had ACR >3.4 mg/mmol. Five Aboriginal children had ACR>25 mg/mmol.
Proteinuria: Proteinuria (excluding trace) was found in 20% of Aboriginal adults and 8% of non-Aboriginal adults. There was a trend for higher rates in non-Aboriginal than Aboriginal girls (16% v 7.5%, p=0.096)
Haematuria: On POC urinalysis haematuria (excluding trace) was an unexpectedly common finding: 35.8% of Aboriginal women, 20.7% of non-Aboriginal women (p=0.004), 15.6% of Aboriginal men and 9% of non-Aboriginal men had haematuria. Haematuria (excluding trace) was found in 20.6% of Aboriginal girls, 13.7% of non-Aboriginal girls (p=0.32), 14.4% of Aboriginal boys (14.4%) and 4 of 45 non-Aboriginal boys (p=0.46). There was no significant difference between boys and girls (p=0.10). Apart from female gender there were no other significant predictors of haematuria (Aboriginality, obesity, age, hypertension, skin infection, ear infection, elevated ACR).
Urine pH: Low urine pH was a common finding: 77.4% of Aboriginal and non-Aboriginal adults and 64.2% of children had urine pH of 6 or less; there was no difference in this finding between Aboriginal and non-Aboriginal people.
Town versus community
There was no difference in the prevalence of one or more elevated urinary markers for kidney disease (haematuria, proteinuria and/or elevated ACR) for adults or children living in the towns compared to those living in remote communities. Fifty-five percent of Aboriginal adults and 27.3% of non-Aboriginal adults living in town had at least one urinary marker of kidney disease compared to 46.4% of Aboriginal adults and 26.1% of non-Aboriginal adults living in remote communities (Aboriginal adults p=0.16; non-Aboriginal adults p=1). The WDKHP found 24.3% of Aboriginal children and 28.4% of non-Aboriginal children living in town had at least one urinary marker of kidney disease compared to 27.7% of Aboriginal children and one in seven non-Aboriginal children living in remote communities (Aboriginal children p=0.54; non-Aboriginal children p=0.67).
Comparison data for children are difficult to find but the ABS data suggest that 19% of Aboriginal adults6 will have at least one marker of kidney disease and the rate for Aboriginal adults in remote areas may be up to 34%6.
Risk factors for renal disease and diabetes
Table 3 shows the multivariate analysis of risk factors for the presence of biomarkers associated with T2DM and renal disease. For adults Aboriginality was a significant predictor for the appearance of all biomarkers. Risk of having diabetes was associated with Aboriginality, obesity and increasing age but there was no independent gender effect. The risk of having at least one biomarker of kidney disease was associated with Aboriginality and female gender but not independently with hypertension or obesity. Sampling occurred year round and environmental temperatures were 0–45ºC. There were no differences between communities, suggesting no association with environmental temperatures. Abnormalities on urinalysis were found even in the youngest children in this study.
Table 3: Western Desert Kidney Health Project results of multivariate analysis of risk factors and biomarkers of renal disease and type 2 diabetes in adults†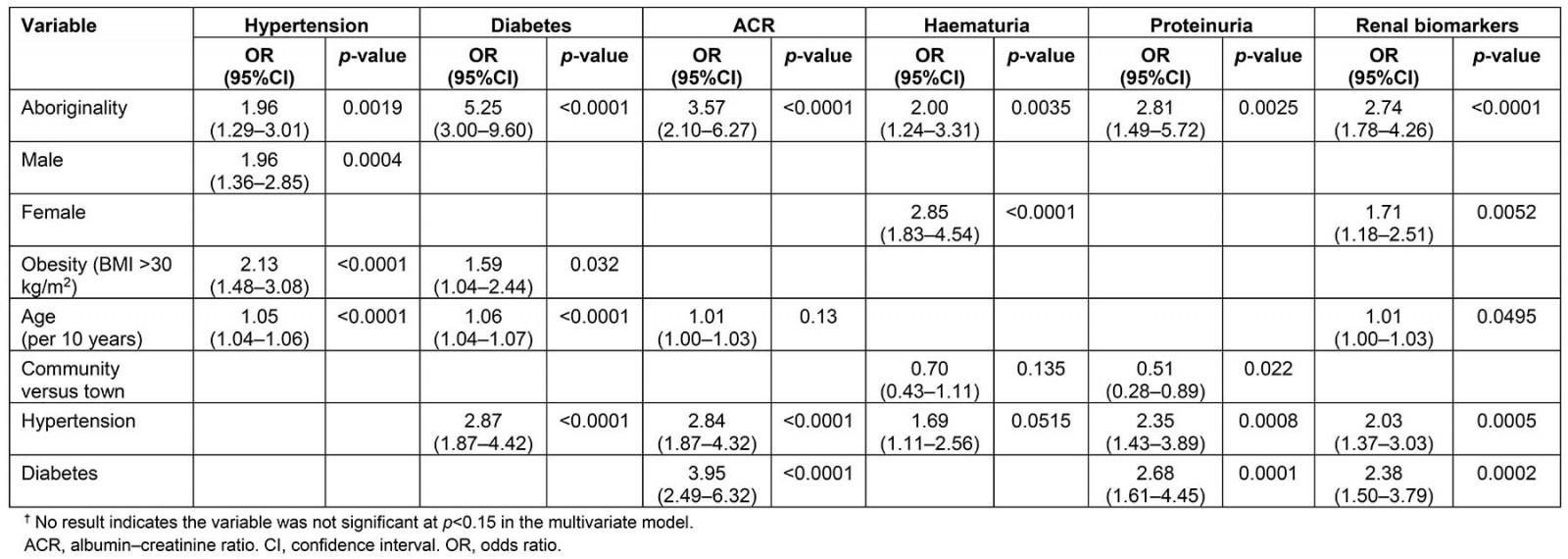
Discussion
The development of renal disease and T2DM is likely to be multifactorial, and the early onset in some population groups suggests factors occurring during childhood, infancy and even before birth are important. Clinical indicators of the early onset of the disease process can be helpful in finding opportunities for intervention. This study used clinical measures with POC testing to look for early indicators of risk and found higher than predicted rates of T2DM and markers for kidney disease in both Australian Aboriginal and non-Aboriginal participants. The difference in rates of disease between Aboriginal and non-Aboriginal people was not nearly as marked as expected – this was interesting given that almost all the Aboriginal participants had spent most of their lives in rural or remote areas, but 91.5% of non-Aboriginal adult participants had grown up in urban settings and many were temporary residents. Ethnicity, therefore, might be less important than location or environmental factors.
The participants in this study might represent those subject to a ‘perfect storm’ of risk factors with exposure to novel environmental factors plus clustering of well known risk factors2. The towns and communities in this study fall into the ‘remote’ (Remoteness Area RA4) or ‘very remote’ RA5 groups and the lowest two Socio-Economic Indexes for Areas (SEIFA) score groups for relative disadvantage9. Poverty and remoteness might limit access to mitigating factors such as fresh foods, especially fruit and vegetables, work and exercise opportunities20,21.
Hyperinsulinism and progression to T2DM have been reported to be particular problems for Australian Aboriginal people and they start at a younger age than non-Aboriginal people22. Acanthosis nigricans is associated with T2DM, insulin resistance and impaired insulin response12,23. The WDKHP detected acanthosis nigricans in very young children and children of normal weight, suggesting that hyperinsulinism is occurring very early. Hyperinsulinism and T2DM contribute to the development and progression of other risk factors for renal disease – particularly hypertension, albuminuria and vascular disease24.
Hypertension is a risk factor for renal disease and contributes to increased mortality and morbidity in end-stage renal disease – so is both a cause and an effect of renal disease25. It has been significantly and directly related to micro-albuminuria, which is an early indicator of renal disease26. This relationship has been demonstrated in the Aboriginal community27. National figures predicted that about 23% of Aboriginal and non-Aboriginal adults would have hypertension; the WDKHP found higher rates than expected for adults28,29. Little information is available about the prevalence or patterns of hypertension during childhood in Australia, although some studies in Indigenous communities have suggested hypertension is a problem beginning in childhood, with up to 12% of children having hypertension28,30-32. The WDKHP found the rate of hypertension in children was similar to other studies28.
Albuminuria, measured as ACR, was found even in very young children in this study and at higher rates than expected, especially for non-Aboriginal adults. Albuminuria has been found to be associated with other risk factors for renal disease: skin infection, a history of post-streptococcal glomerulonephritis, obesity, hypertension, hyperinsulinism and T2DM33. Albuminuria during childhood may be transient but there is little information about whether transient albuminuria is associated with increased risk of renal disease, although in a high risk population albuminuria in childhood may be associated with disease later in life24.
The present study revealed a higher rate of haematuria than predicted – 20% of people tested had haematuria (greater than trace). The cause and significance of this are not clear but a contributing factor may be exposure to toxins. Haematuria can occur as a result of inflammation, irritation or disruption of the uroepithelium anywhere along the renal tract. Nephritis (inflammation of the kidney), particularly following streptococcal disease, may be a significant risk factor for renal disease later in life34. Rates of skin infection in this study were lower than studies in Northern Australia where up to 48% of Aboriginal children have been reported to have skin infection35.
Environmental toxins might be important irritants of the urinary tract, causing haematuria. The drinking water in most of the study communities is heavily contaminated with nitrates and, in at least one community, uranium36,37. The WDKHP found that 30% of people in that community had haematuria greater than trace and a further 24% had a trace of blood detected in their urine – a total of 54% of people having some degree of haematuria, suggesting irritation or inflammation in the renal tract. One of the effects of uranium ingestion is renal inflammation and damage, and this is exacerbated by the presence of nitrate and the formation of uranyl nitrate38,39.
More than 70% of participants in the WDKHP were found to have a urine pH of 6 or less, suggesting a renal response to metabolic acidosis. An acid load will result in metabolic acidosis as the body strives to maintain blood pH at all costs. Buffers are used initially and then renal excretion of acid (hydrogen ions) increases. Urine pH decreases but urine pH cannot decrease to less than about 5. When this limit is reached, urate and ammonia are formed to ‘mop up’ excess hydrogen ions40,41. Metabolic acidosis will stimulate the kidney to excrete hydrogen ions through the action of glutaminase and other enzymes in the renal tubules. The activity of these enzymes is governed, at least in part, by increased pituitary adrenocorticotropic hormone production. The increase in this hormone will result in increased cortisol and aldosterone production. These hormones act on the renal tubules to increase ammonia production to facilitate hydrogen ion loss. Excess cortisol promotes an increase in visceral fat, reducing the effectiveness of insulin and contributing to the development of insulin resistance and T2DM42. Aldosterone will stimulate the kidney to retain sodium and cause hydrogen ion and potassium loss43. In the kidney, metabolic acidosis results in a decrease in urinary citrate, increased urinary production of urate and ammonia and increased risk of renal stones. Renal stones, particularly uric acid and oxalate stones, have been found to be a particular problem in Australian Aboriginal people, especially children, from remote areas44,45.
Metabolic acidosis affects mitochondrial function in cells and can shorten mitochondrial life, resulting in shortened cell life. The acid load is concentrated in the kidneys, which will affect kidney cells in particular – contributing, ultimately, to renal failure40,41,46. This can be exacerbated by other factors such as low birth weight and small kidney size at birth, urinary and systemic infections, and stress with changes in cortisol–insulin–adrenalin pathways, smoking, diabetes and metabolic syndrome.
Metabolic acidosis causes a decrease in total body potassium and this will stimulate insulin secretion47. Metabolic acidosis, even relatively mild degrees of acidosis, reduces skeletal muscle sensitivity to insulin, contributing to insulin resistance48. Acidosis inhibits protein synthesis, which may increase the activity of growth factors and protein kinase c, increasing insulin-like growth factor, contributing to insulin resistance48,49.
An important question, therefore, is why people living in this remote area would have chronic metabolic acidosis. Heat stress has been suggested as a factor in chronic kidney disease in remote areas but this study found no difference in risk factors with changes in environmental temperatures. It is likely to be multifactorial, but water quality and the presence of potentially toxic contaminants, such as chloramine, nitrate50,51 and uranium38, is a problem for many remote towns and communities, including those in this study36-38. The water from bores in the area is naturally contaminated with uranium and nitrate and often not filtered36,52. For most areas information on uranium levels in drinking water is not available.
For many years, including during this study, chloramine has been added as a disinfectant to municipal water supplies in the study region37,52. Chloramine, or ammonia left in the water after dissociation, in the presence of chlorine, may convert to ammonium chloride. Ammonium chloride is used therapeutically in the treatment of severe metabolic alkalosis to reduce the pH of the blood, so it can cause metabolic acidosis53.
Nitrates in drinking water can come from natural sources as a result of breakdown of organic material leaching into the groundwater and from the use of fertiliser. Many of the towns and communities in remote Australia are at risk from drinking water taken from bores that are contaminated with naturally occurring nitrates. For at least the last 10 years, 11 regional towns in Western Australia have been exempt from the drinking water safety guidelines due to the high level of nitrate contamination37,50,52. Many remote Aboriginal communities have only unfiltered or poorly filtered bore water, with nitrate levels in drinking water far in excess of the safety guidelines36,38. Nitrate and nitrite ingestion in food and water can contribute to metabolic acidosis as well as having effects through the actions of nitrosamines50. Nitrate and nitrite ingestion is associated with increased risk of many health effects including cancer, thyroid disease, T2DM, birth defects, non-alcoholic steatohepatitis (fatty liver), Alzheimer’s disease, hypertension and cardiovascular disease51,54-56. The effects on acid–base balance, diabetes, hypertension and cardiovascular disease are likely to contribute to the risk of renal disease.
The role of drinking water quality in chronic disease is of interest internationally, with nitrate of particular interest51,56-58. In many places isolated nitrate contamination in drinking water is the result of fertiliser use but in Australia the natural presence of nitrate in ground water facilitates the leaching of other contaminants, such as uranium and other heavy metals, from underground deposits into the water38,51,59. High nitrate levels in drinking water are a marker of inadequate filtration so the risk of heavy metal contamination is significant. As discussed, some remote Aboriginal communities in Western Australia are known to have unacceptable levels of uranium in the drinking water but information about other potentially toxic contaminants is not currently available36,38,52.
Strengths and limitations
The geographical and climatic challenges to conducting this study were great. However, despite small population sizes, high participation rates were ensured because of the extensive recruitment strategies employed. There was good participation from Aboriginal people and others who were exposed to the engagement strategies and came into direct contact with the study team, especially in the remote communities. The mobile clinics operated during daylight hours on weekdays, perhaps limiting participation of people living in the towns but working on mine sites and pastoral stations in the surrounding area, biasing the results particularly for the non-Aboriginal people. People working in the mines often work at mine sites at some distance from their home (fly in, fly out or drive in, drive out) and tend to stay at the mine site for the duration of their ‘swing’. Thus, although counted in the ABS data, they were not present to participate in the study, reflected in the lower participation rate in the towns.
The unemployment rate for Aboriginal people in the area is very high, at least three times higher than for non-Aboriginal people, enabling higher participation from Aboriginal people60,61.
The climatic challenges caused an unexpected, and unavoidable, small, amount of data loss. Unfortunately repeating these clinical tests was not possible.
The participation of Aboriginal and non-Aboriginal people living in similar environmental and social conditions is important and has revealed that there is more similarity than expected, perhaps providing an opportunity to examine factors that might be lost in larger studies.
Conclusion
The WDKHP found higher than expected rates of markers for T2DM and kidney disease compared with national quoted figures for Aboriginal and non-Aboriginal adults and children, with Aboriginal women the highest risk group. There was no difference between participants living in regional towns and those living in remote Aboriginal communities. The rates for non-Aboriginal participants were, in general, much higher than expected. Exposures in common may be more important than ethnicity. The high prevalence of aciduria and haematuria found in this study suggest that factors contributing to a chronic metabolic acidosis and inflammation or irritation of the urinary tract need to be explored – and drinking water quality in this remote area may be a contributing factor. Many of the contributing factors are potentially modifiable – such as water quality, food supply, exercise opportunities and living conditions – offering scope for interventions to reduce the risk and burden of these diseases.
Acknowledgements
Western Desert Kidney Health Team: Annette Stokes, Christine Jeffries-Stokes, Lachlan McDonald, Rachel Burgess, Andrew Hughes, Ruth Kelly, Samuel Stokes, Robin Kelly, Pearl Scott, Albert McKenzie, Adrian Schultz, Elizabeth Huriwai, Sharon Evans, Craig Sinclair, Emma Lalor, Katherine Stokes, Nick Larkins, Gemma Baines, Jeanne Daly, Linda Anderson, Priscilla Robinson and the staff of the Rural Clinical School of the University of Western Australia.
Communities: Laverton, Mt Margaret, Mulga Queen, Leonora, Menzies, Kurrawang, Coolgardie, Norseman, Coonana, Coolgardie, Tjuntjuntjarra and Wongutha Birni Aboriginal Corporation.
Artists: Steve Aiton, Ken Allen, Clare Bailey, Alison Dimer, James Gentle, Catherine Howard, Francis Italiano, Peter Keelan, Ruth Koedyk, Martin Meader, Phillipa O’Brien, Evelyn Roth, Suri Bin Saad, Matt Scurfield, Nalda Searles, Leticia Shaw, Rodney Stratton, Poppy Van Ord Granger and Cecile Williams.
Volunteers: Chong Chui Han, Ruth Monck, Lorraine Sholson, Geoffrey Stokes, Rebecca Stokes, Ada Stokes, Milly-anna Stokes, Tom Volkman, Anna Wills, Khiang Wai and the Students of the rural clinical schools of the University of Western Australia, University of Notre Dame and University of Tasmania.
Delivery partners: Rural Clinical School of WA, University of Western Australia, University of Notre Dame, Bega Garnbirringu Health Services Corporation, Goldfields Midwest Medicare Local and West Australian Country Health Service.
Funding: Australian Government Department of Health and Aging, The Australia Council for the Arts, Western Australian Department for Culture and the Arts, Healthway – The Health Promotion Foundation, Royalties for Regions, Lotterywest, BHP Billiton Nicklewest, JT Reid Foundation, Southern Cross Goldfields Ltd, Cape2Cape Motorcyclists.
Academic support: Jan Payne, Carol Bower, Deborah Lehmann, Shantidani Minz, Campbell Murdoch and Phil Reid.
References
You might also be interested in:
2013 - Pilot project and evaluation of delivering diabetes work-based education using video conferencing
2006 - Medical students' assessments of skill development in rural primary care clinics
