Introduction
As of 2 July 2021, there have been almost 183 million cases and nearly 4 million deaths globally from severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2; COVID-19). Of these, nearly 34 million cases and over 605 000 deaths have been in the USA1. Rural areas of the USA have higher rates of older patients and patients with several medical comorbidities2,3. This, coupled with a severe and chronic shortage of medical subspecialists3, relative shortages of both hospital beds and intensive care unit (ICU) beds4 and the accelerating rate of financial failure of many rural hospitals5, has left a large part of the rural USA without sufficient hospital bed capacity, personal protective equipment, medications or access to subspecialists to treat some of the sickest patients with COVID-19.
Rural hospitals also face another under-appreciated but very significant challenge in treating COVID-19 patients. In most rural hospitals in the USA, there is a lack of the experience and/or infrastructure necessary to participate in clinical trials6,7. Early in the pandemic, rural physicians without reliable access to trials were left with the choice of either treating their COVID-19 patients with off-label medications typically recommended for use only in the context of clinical trials8,9, or using supportive care alone. Thus, as news of the COVID-19 pandemic overwhelming New York City’s hospitals captured the world’s attention in March 2020, an overlooked story became that of the many rural areas in and outside of New York state that were also struggling with COVID-19 caseloads, under-resourced local health systems, limited medical resources and an inability to rely on already strained regional academic centers. In the present case, this 'perfect storm' provided the impetus to develop an inpatient COVID-19 treatment team as well as a durable pandemic response system for the authors’ rural area.
New York State is 86% rural land, and 18% of its residents live in rural areas. As with most of rural America, New York’s rural communities are largely underserved, and to this end the 2019 rural access to primary care in New York State report revealed that non-core rural areas in New York have less than one quarter of the number of primary care providers per capita than metropolitan and micropolitan communities in the state. St Lawrence County is the largest county in New York State and shares its northern border with Ontario, Canada. The county population is about 112 000, 94% White and 13.5% of people aged 65 years or more. In the county, 17.8% of residents currently live below the poverty line10. St Lawrence Health (SLH) serves a catchment area of roughly 200 000 people across four rural counties. It includes three hospitals, one of which is a critical access hospital and another of which has just 25 beds. The flagship hospital of SLH is Canton Potsdam Hospital (CPH), which has 94 beds, a critical care unit and an emergency department. CPH is also a Level III Trauma center and offers services for 25 different specialties (Fig1)11. A sharp increase in the number of medical subspecialists recruited to CPH over the past 6 years has been one of the major changes in the authors’ region, and it was because of this that SLH was able to recruit an infectious disease physician, physician assistant and a rheumatologist with experience in treating interstitial lung disease to its inpatient COVID-19 treatment team. This kind of subspecialty support is increasingly rare in rural communities, but this team still lacked critical resources, including no access to remdesivir or clinical trials in the first phase of the pandemic and no access to dialysis services or extracorporeal membrane oxygenation as well as limited surge capacity at CPH.
This article aims to describe SLH’s model for inpatient COVID-19 care delivery focusing on its hub-and-spoke model for transferring patients to its flagship hospital, its development of a small team for treating hospitalized patients and its collaboration with its own Department of Clinical and Rural Health Research to provide clinical trial opportunities for its community. In the context of this care delivery system, it aims to show how a small rural health system with less than 150 beds across its three hospitals was able to build on the momentum of several key decisions made before the pandemic (in particular, the development of both an increased base of medical subspecialists and a new research department) in order to strengthen its pandemic response system.
This article also aims to describe the demographic characteristics and clinical course of the 20 COVID-19 patients admitted to CPH over the first 2 months of the COVID-19 pandemic. This time period was selected because it is the phase of the pandemic in which SLH had no access to clinical trial opportunities, and in turn is a good representation of resource challenges faced by other rural hospitals and health systems in the USA. St Lawrence County ultimately saw a dramatic spike in cases beginning in November of 2020, peaking in January 2021 and beginning to subside in March 2021 (Fig2)12. As of 17 March 2021, St Lawrence County had had 6451 cases, with 92 deaths since the onset of the pandemic13. The final aim of the article is to discuss strategies for implementing similar care delivery strategies in other rural areas both during this and future pandemics.
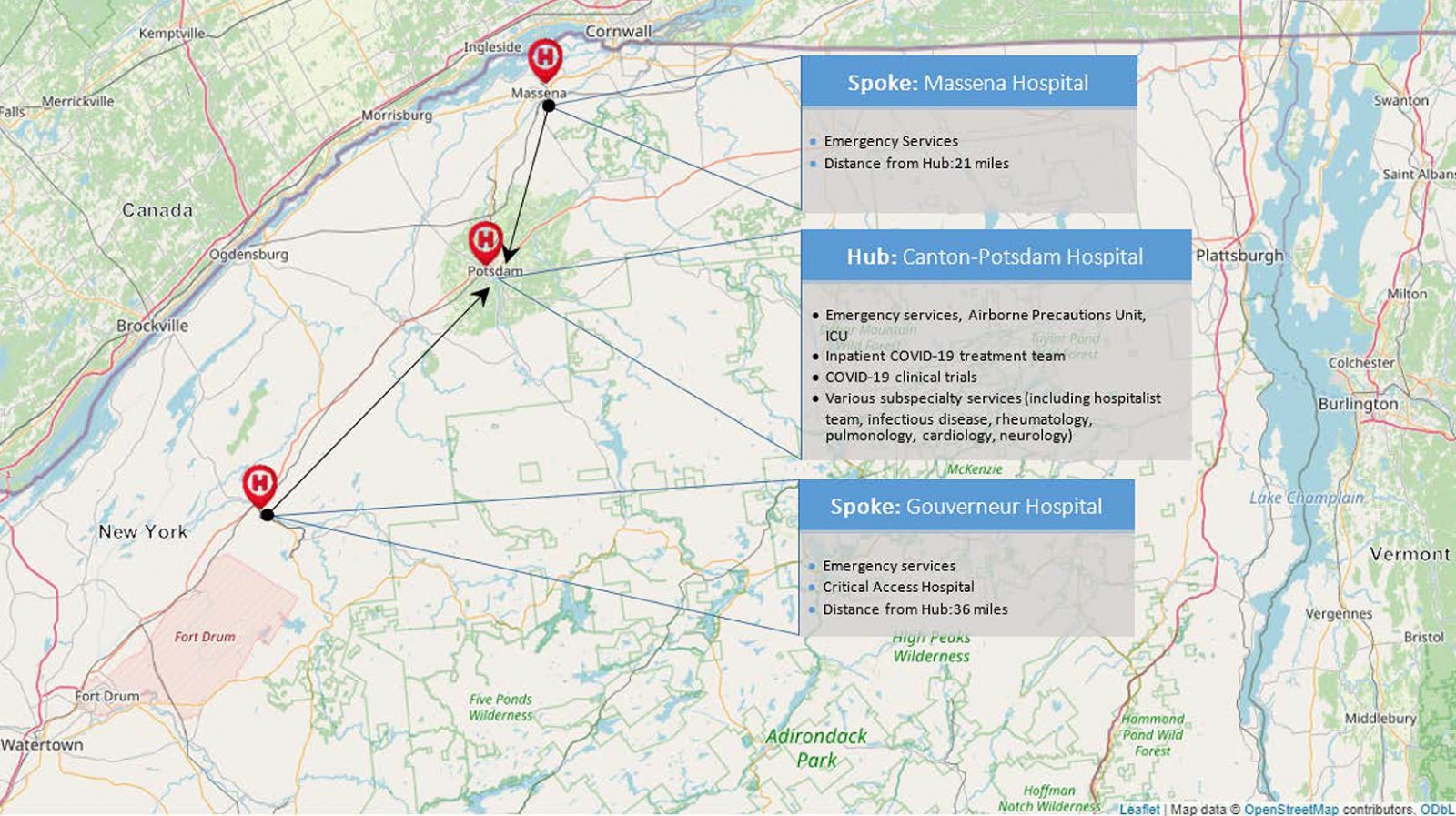 Figure 1: Map of the three hospitals that make up St Lawrence Health in Northern New York, including each hospital’s main services as they relate to COVID-19.
Figure 1: Map of the three hospitals that make up St Lawrence Health in Northern New York, including each hospital’s main services as they relate to COVID-19.
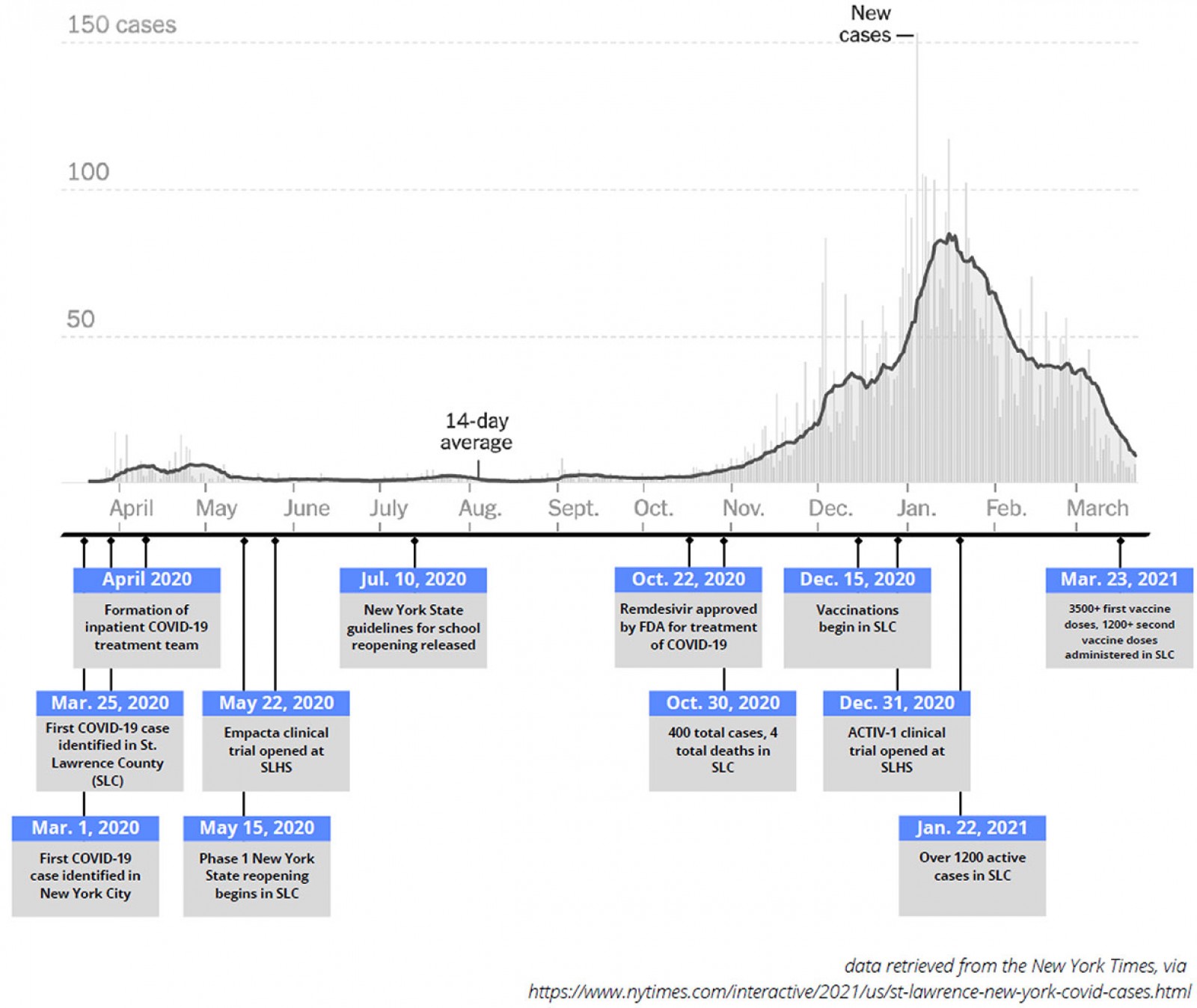 Figure 2: Timeline of key milestones below a graph of new COVID-19 cases in St Lawrence County (SLC) during the pandemic, March 2020 to March 2021.
Figure 2: Timeline of key milestones below a graph of new COVID-19 cases in St Lawrence County (SLC) during the pandemic, March 2020 to March 2021.
Methods
As part of its COVID-19 triage plan, SLH uses a hub-and-spoke model in which all COVID-19 patients requiring admission are transferred to its flagship hospital, CPH. As an example, a COVID-19 patient in the emergency room of a smaller (spoke) hospital within SLH would be transferred to CPH, admitted to its airborne precautions unit and, when necessary, be admitted or transferred to the ICU (Fig1).In order to meet this increased clinical demand, SLH assembled a clinical team consisting of an infectious diseases physician and advanced practice provider, a rheumatologist with experience in managing interstitial lung disease, a hospitalist team with experience in critical care medicine and an antimicrobial stewardship pharmacist with experience in general inpatient pharmacy. Since its inception, this team has met daily to conduct multidisciplinary team rounds for all hospitalized COVID-19 patients at CPH. As part of these rounds, the team also discusses COVID-19 research updates, epidemiologic data updates for the region and COVID-19 medication inventory updates for the pharmacy. The date of data censoring for this study coincides with the date on which SLH began its first inpatient COVID-19 clinical trial at CPH, and to this end the SLH inpatient COVID-19 treatment team’s daily rounds also include a discussion of both new and existing COVID-19 trial opportunities through SLH’s clinical research department.
This study was conducted at CPH during the initial phase of COVID-19 infection in St Lawrence County between 20 March and 22 May 2020. All consecutive adult (age ≥18 years) patients with confirmed and active COVID-19 infection who were admitted to CPH over the dates of the case series were included. A confirmed active COVID-19 case was defined as having a positive RT-PCR assay on a specimen obtained by nasopharyngeal swab and sent to a reference lab for testing for SARS-CoV-2 RNA14. Patients with positive serologic but negative RT-PCR testing at the time of admission were excluded. Twenty adults were identified, none of whom were pregnant or prisoners. A waiver of consent was obtained for patients lost to follow-up. All other patients provided written authorization to use and share health information. Clinical outcomes were monitored until the final date of follow-up on 22 May 2020.
Data were collected from CPH’s electronic medical record system (Meditech and eClinicalWorks). Data collected on admission included demographic information, baseline comorbidities, symptoms prior to admission, and pertinent travel and COVID-19 exposure histories. Data collected over the course of the hospital stay included vital signs, lab results, chest imaging results, echocardiogram and electrocardiogram results, inpatient medications and a daily National Institutes of Health (NIH) ordinal scale score for all patients.
Measured clinical outcomes included length of stay and death rate. Clinical measures included severity of illness, supplemental oxygen requirements, need for invasive mechanical ventilation, use of vasopressors, acute kidney injury (defined as an increase in serum creatinine of ≥0.3 mg/dL within 48 hours or ≥50% within 7 days or urine output of <0.5 mL/kg/h for >6 hours15) and transaminitis.
Statistical analysis was performed using R software v3.3.2 (R Project; https://cran.r-project.org/bin/windows/base/old/3.3.2). Results were reported as means and standard deviations, medians and interquartile ranges or, in the case of categorical variables, counts and percentages. There were no missing data for which imputation was necessary.
Ethics approval
The study was approved by the SLH Institutional Review Board (202063).
Results
Demographics and baseline clinical characteristics
The demographic and baseline clinical characteristics of the 20 COVID-19 positive patients admitted to CPH between 20 March and 22 May 2020 are shown in Table 1. The median age of the patients was 63 (interquartile range (IQR) 51–74) with 12 (60%) females. Ten (50%) patients were obese, four patients (20%) had cardiovascular disease, two (10%) had diabetes mellitus, four (20%) had pulmonary disease (COPD or asthma) and four (20%) had obstructive sleep apnea. Six (30%) patients were current or former smokers. The mean Charlson Comorbidity Index was 3.2 (standard deviation (SD)±2.3).
The mean duration of symptoms before admission was 6.4 days (SD±6.22, range <1–25) and one patient had a history of travel to an endemic country in the month prior to admission. Fourteen patients (70%) had contact with a known COVID-19 positive patient prior to admission. The most common symptoms upon presentation included cough (55%), shortness of breath (55%), and/or fever (subjective or objective, 50%). Additional symptoms upon presentation are shown in Table 1.
On admission, six (30%) patients had a measured temperature of ≥38.0ºC(100.4ºF), three (15%) had a heart rate ≥100 beats per minute, 12 (60%) had a respiratory rate ≥20 breaths per minute, and 9 (45%) had an oxygen saturation ≤93%. Seven (35%) patients were receiving supplemental oxygen when the oxygen saturation level was measured on admission.
Table 1: Demographics and baseline clinical characteristics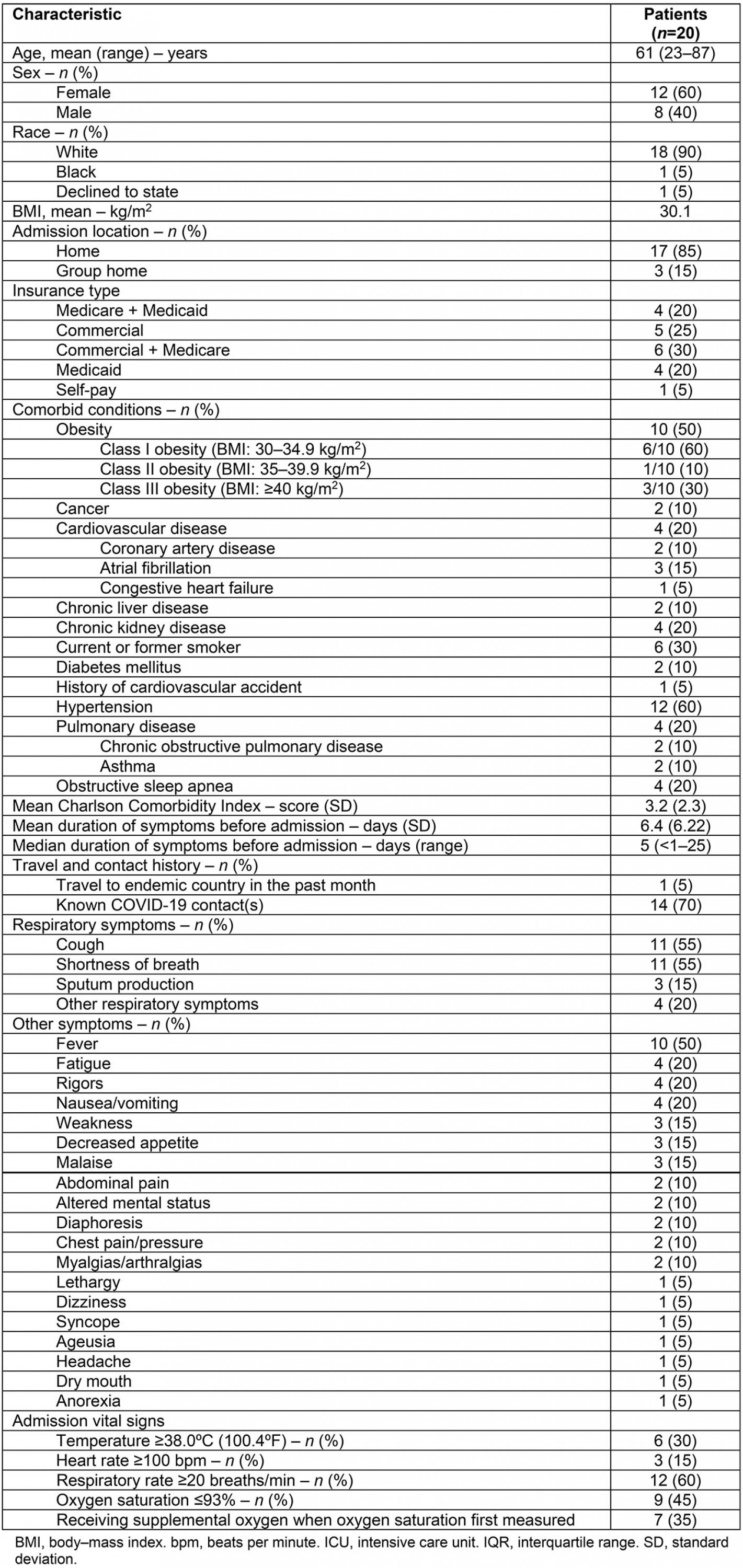
Radiologic and laboratory findings
The radiologic and notable laboratory findings of these patients are described in Table 2.
Fifteen (93%) patients displayed radiographic evidence of COVID-19 pneumonia on chest X-ray or CT. Of these, four (27%) patients had diffuse, severe bilateral alveolar infiltrates on chest X-ray.
On admission, ferritin and lactate dehydrogenase median values were elevated at 599 ng/mL (IQR 372–949, reference range 11-264) and 291 U/L (IQR 266–369, reference range 84–246), respectively. The median lymphocyte count was at the lower limit of normal range at 1000/mm3 (IQR 800-1125, reference range 1000–3100). Over the course of hospitalization, the median absolute lymphocyte count decreased (800/mm3, IQR 600–1000) and the median ferritin level (1026 ng/mL, IQR 594–1336) and lactate dehydrogenase level (334 U/L, IQR 294–519) increased.
Troponin was elevated in one patient (5%) on admission and three patients (15%) over the course of hospitalization. Additional admission and hospitalization laboratory findings are included in Table 2.
Table 2: Radiologic and baseline laboratory findings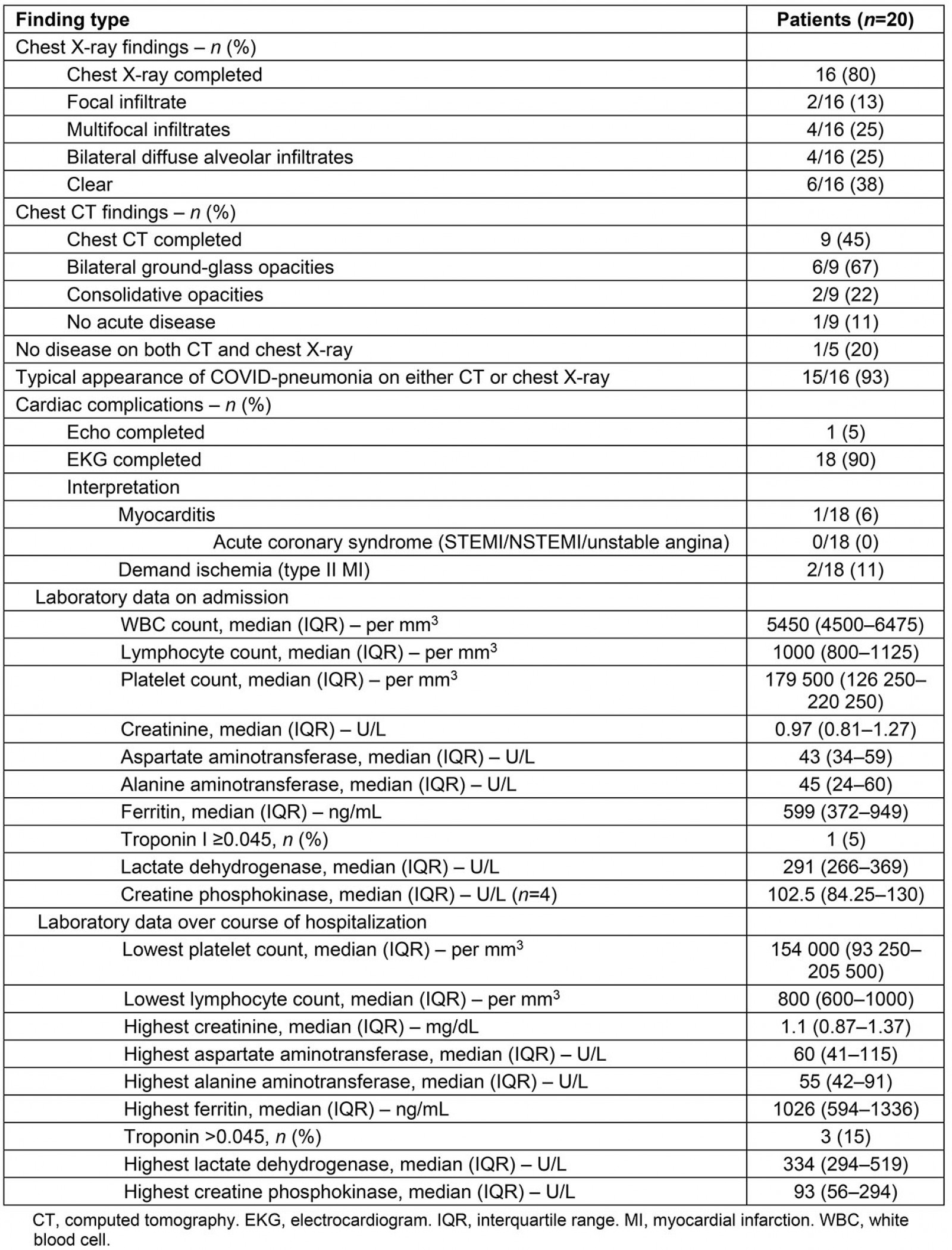
Clinical course, disease severity, and treatment course
The clinical course, disease severity, and treatment course of these patients is described in Table 3. The median length of stay was 6 days (IQR 2–23). Four patients (20%) required ICU level care with two patients requiring ICU care for 1 day and two patients requiring ICU care for 19 and 27 days, respectively.
Using the NIH COVID-19 severity of illness categories16, nine (45%) patients met NIH criteria for severe illness and seven (35%) met criteria for critical illness. Of the seven patients with critical illness, all (100%) met criteria for respiratory failure, three (43%) developed septic shock, five (71%) developed multi-organ dysfunction, and six patients (86%) developed acute kidney injury15. Sixteen (80%) patients developed transaminitis.
Cardiac complications included demand ischemia in two (10%) patients and myocarditis in one (5%) patient. No patients developed acute coronary syndrome and there were no other thromboembolic complications.
Seven (35%) patients did not require supplemental oxygen, five (25%) received low flow supplemental oxygen, three (15%) received high flow oxygen via high flow nasal cannula, three
(15%) received non-invasive mechanical ventilation, and two (10%) received invasive mechanical ventilation. No patients were proned.
Five patients (25%) received hydroxychloroquine, two (10%) received azithromycin (in addition to hydroxychloroquine), four (20%) received systemic corticosteroids, nine (45%) received tocilizumab, and three (15%) received convalescent plasma. Of the patients who received tocilizumab, the mean number of doses per patient was two (SD ±0.74). Five patients (25%) received inhaled bronchodilators and three (15%) received vasopressors.
The mean NIH ordinal scale score was 4.3 on admission (SD ±0.78), with the mean lowest score over the course of hospitalization being 3.8 (SD±1.2). One (5%) patient met criteria for invasive mechanical ventilation, but this was not consistent with their goals of care. The patient died after transitioning to comfort measures. The remaining 19 (95%) patients survived and no patients were transferred to other hospitals.
Table 3: Clinical course and disease severity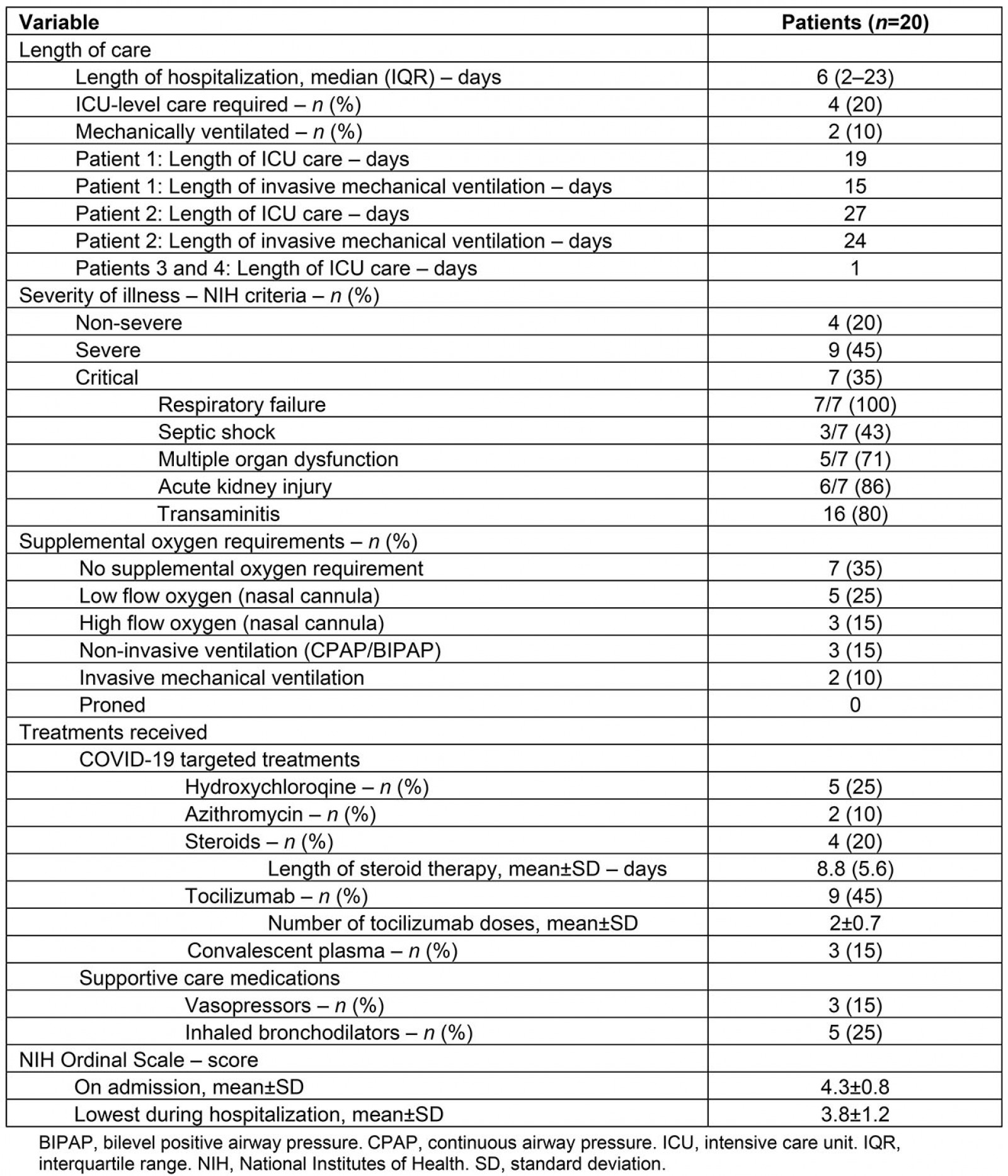
Discussion
Clinical approach and challenges
This regional, single-center study represents to the authors’ knowledge the first template for delivering care to hospitalized COVID-19 patients in a rural area. SLH’s approach included the creation of a regional hub-and-spoke system for routing patients to its flagship hospital as well as creation of a multidisciplinary team with experience in hospitalist medicine, rheumatology, infectious diseases, and inpatient pharmacy. Since the start of the pandemic, involvement on this team has been voluntary rather than compulsory. Furthermore, the relative lack of subspecialists in this rural region (there is, for example, just one rheumatologist and one infectious disease physician in St Lawrence County) has meant that team members have often had to work long hours over the course of the pandemic. Lack of subspecialty support is a very typical rural problem, and in turn this mission-driven (rather than directive-driven) approach has been one of the critical components of the SLH team’s success.
Although the standard of care for treating COVID-19 has evolved and continues to evolve, the core elements of SLH’s pandemic response system have remained intact since its COVID-19 treatment team was first formed in March 2020. This system has allowed SLH and its inpatient COVID-19 treatment team to continue to centralize a number of limited resources (clinical expertise, personnel, medications), to stay abreast of developing research on SARS-CoV-2 infection, to participate in a number of high profile COVID-19 clinical trials and to quickly adapt to changing guidelines for the treatment of COVID-19. Fortunately, although there was a high rate of comorbidities in this case series, the death rate was 5%, no patients were transferred to outside hospitals and no full code patients died. This was in the setting of lacking access to remdesivir, dialysis services and extracorporeal membrane oxygenation in SLH and also lacking any access to COVID-19 clinical trial opportunities over the dates of this case series.
One of the COVID-19 treatment team’s key challenges has been in continuing to adapt to an often quickly changing standard of care. In this case series, for example, five patients initially received hydroxychloroquine and/or azithromycin, but upon publication of a retrospective Veterans Affairs study that suggested no benefit to this approach17 the team stopped using both of these medications. In light of an encouraging case series from China18 and encouraging data from an open-label randomized controlled tocilizumab trial in France19, the team also used tocilizumab for nine of its patients. This decision was made with verbal consent of patients and only for patients who the team determined to be in the hyperinflammation (cytokine storm) phase of their illness. Furthermore, limited availability of tocilizumab in the first phase of the pandemic during which this case series is described made it at times challenging to procure this medication. In response to this, the COVID-19 treatment team worked closely with SLH’s clinical research department to develop clinical trial opportunities for its hospitalized COVID-19 patients, and this case series concluded at the starting point of SLH’s first double-blind, randomized COVID-19 inpatient trial (Fig2).
Today, by contrast, the standard of care for treating COVID-19 has again changed, and it is now common to administer dexamethasone to patients on supplemental oxygen and to use tocilizumab for patients newly admitted to or rapidly progressing towards admission to the ICU20-22. Interestingly and fortunately, the COVID-19 treatment team’s early approach of using tocilizumab around what the team has referred to as the 'clinical inflection point' of COVID-19 (the point at which a patient is either newly into or about to enter cytokine storm) may have been a good instinct, as the team now uses a different although perhaps related metric (being at or close to the 'ICU inflection point') to determine when to use tocilizumab for its COVID-19 patients. Other previously used treatments (such as convalescent plasma) that have not been shown to have a clinical benefit are no longer used by the team, whereas still others (such as treatment dose anticoagulation for selected non-critical patients23 and dexamethasone for patients on supplemental oxygen) that were not used earlier in the pandemic are now routine. Furthermore, whereas the team had no access to remdesivir over the first phase of the pandemic, described in this case series, access to and early use of remdesivir is now routine in SLH as well.
Another obstacle that the SLH inpatient COVID-19 treatment team has continued to face is that of demographics. In this case series, half of patients were obese and three-quarters were either covered by Medicaid and/or Medicare or had no medical insurance. Obesity and poverty are both established COVID-19 risk factors, and with this in mind perhaps tocilizumab is to be credited at least in part for the low mortality rate in this case series.
The team had just one returned traveler from a country in which COVID-19 was endemic in this case series. This highlights a final challenge not only of SLH’s COVID-19 treatment team but for all healthcare providers fighting COVID-19. Namely, transmission tends to occur both within and between communities, and when there isn’t a concerted state or federal effort to prevent the spread of COVID-19, rural places (often because of migrating travelers from regionally affected cities) are bound to be affected. Any rural pandemic response system must also consider this unavoidable fact, and to this end developing better strategies for preventing the spread of viruses (including better and ideally locally delivered public health messages around everything from masks to vaccines) will need to be a part of local, state and federal planning for future pandemics.
Broader applicability
There are several lessons to be learned from SLH’s rural inpatient COVID-19 experience. The first is that small teams of providers can be quickly assembled in response to unexpected medical emergencies and events. This is relevant to rural areas in the USA, the majority of which have local health systems that lack the resources of academic centers24. The nature of these small teams is also important. In particular, physicians who are generalists within their fields and who are willing to work long hours (ie who are mission rather than directive-driven) can be used to meet unexpected needs. In the case of SLH’s inpatient COVID-19 treatment team, a rheumatologist with experience in the management of interstitial lung disease, two hospitalists with experience in critical care medicine, an infectious disease physician, an inpatient pharmacist and a number of advanced practice providers on both the infectious disease and hospitalist teams all served broad roles within their respective fields. These are just several examples, and what is even more important than the team’s COVID-19 experience is the larger lesson that in the setting of resource limitation small teams of generalists can be used to respond to a wide range of medical events.
Another lesson is that a hub-and-spoke system in which a larger rural hospital (the hub) provides the resources to treat the sicker COVID-19 patients in its region can help conserve limited medical resources in a time of crisis. Through such a system, smaller rural hospitals (the spokes) within a region can refer sicker COVID-19 patients to the hub hospital and, in turn, enable those patients to be managed locally (Fig3).
A third lesson is that having the infrastructure and experience to conduct clinical trials is invaluable in a pandemic. At a time when the majority of rural sites lack this infrastructure6,7 there is often a divergence between national treatment guidelines8,9 and the on-the-ground reality for rural hospitals. In SLH’s case, outside of convalescent plasma (which was an open-label Expanded Access Program that did not require prior clinical research experience), SLH was not enrolled in any clinical trials over the dates of this case series. Because of this, and because the authors’ hope is that the SLH COVID-19 treatment team’s early experience can serve as a realistic template to help guide future rural pandemic response efforts, the date of data censoring for this study was chosen as the date on which SLH began its first double-blind randomized clinical trial.
A fourth lesson is that the risk of burnout for medical providers in a pandemic can be particularly high in rural areas. The reasons for this include the strain of working with limited hospital bed capacity, challenges in transferring sicker patients to regional academic centers (ie if those centers have no beds of their own), challenges in implementing national guidelines that may be impractical for rural hospitals (ie recommendations to use certain medications only in the context of clinical trials that are unlikely to reach rural communities) and the strain of often working with limited or no backup. SLH’s example shows again here the value of working in small teams in which, in exchange for longer hours, there is the familiarity and comfort of working with colleagues who one knows well and with whom one can share both the sorrow of losing a patient and the joy of watching a patient pull through.
A fifth and overarching lesson is that crisis management either in or out of a pandemic requires adequate networks of care. Such networks must include systems for centralizing and streamlining healthcare resources, good communication between physicians and advanced practice providers at hub-and-spoke hospitals, clinical research infrastructure and expertise, and ideally strong relationships between rural and regional academic physicians25,26.
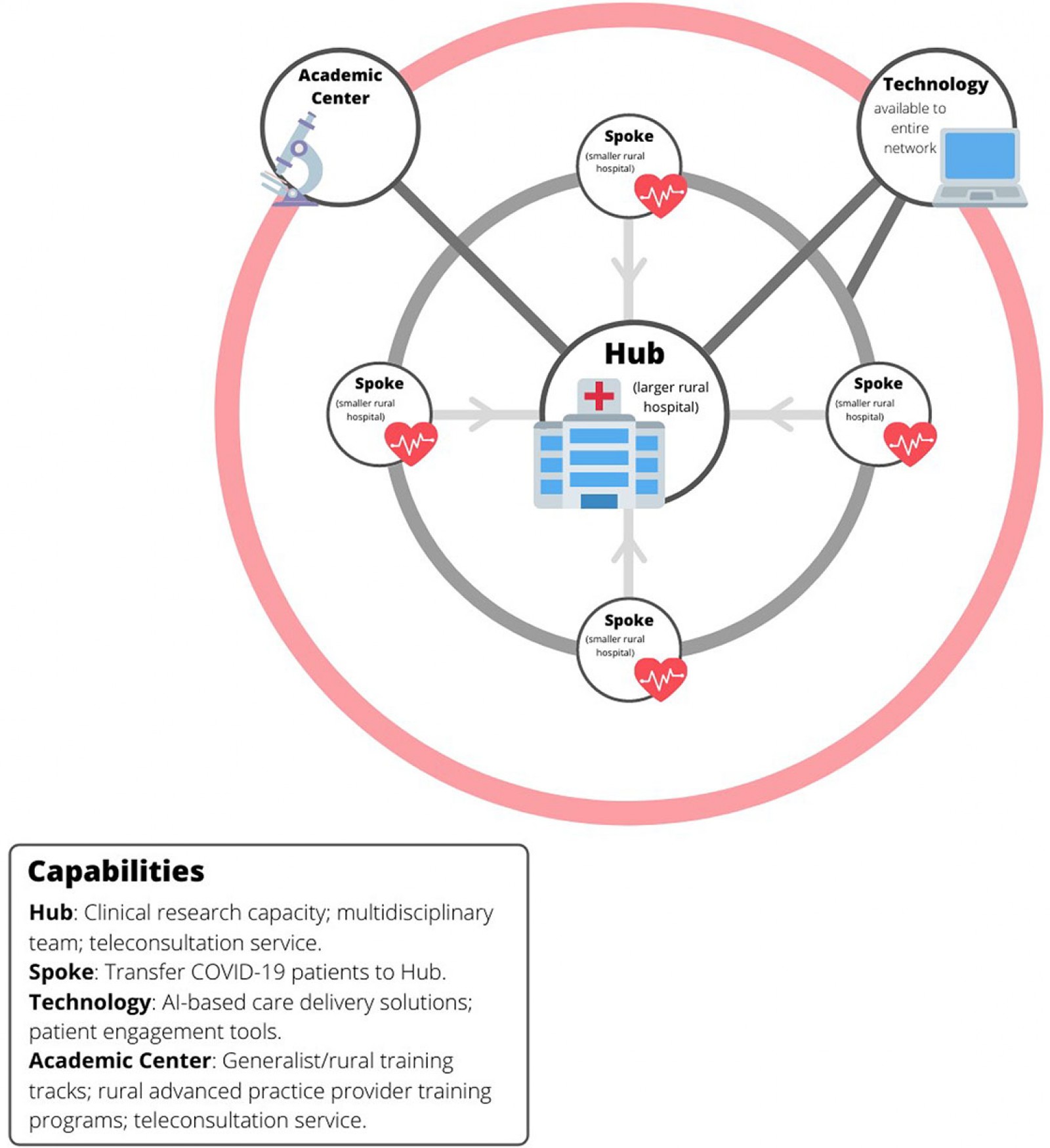 Figure 3: Hub-and-spoke model for rural care delivery.
Figure 3: Hub-and-spoke model for rural care delivery.
Limitations
This case series has several important limitations. These include its small sample size, the lack of a control group, the retrospective nature of this series and the relatively small numbers of patients who received immunomodulatory medications. It should also be noted that SLH’s rural COVID-19 experience has been atypical. Whereas in SLH there exists the subspecialty expertise and access to medications to at least approximate clinical trial-like conditions for its patients, the majority of rural health systems lack such resources. Yet SLH serves one of the most vulnerable populations to COVID-19 in New York State27 and the patients in this series provide at least a reasonable (if small) representation of the rural US population28. As this is to the authors’ knowledge only the second reported case series of hospitalized COVID-19 patients in a rural area (in both the USA and the world)29, the authors believe that their experience can help provide a template through which to structure future crisis management approaches in rural areas. Furthermore, while this case series may not reflect the typical resources of a rural hospital, the authors also believe that such resources can be developed. In SLH’s case, it has been able to create a clinical research department and recruit the subspecialists on its COVID-19 inpatient management team within the past 5 years.
Conclusion
A potential rural pandemic response system of the future might begin with a hub-and-spoke system in which sicker patients are routed to a central, or hub rural hospital (Fig3). This has been the model for COVID-19 care delivery in this region, and the authors’ own experience suggests that, with the right impetus, the resources for such a model can be developed. An improved version of this model would also include a backup COVID-19 consultation team at a regional academic center, the incorporation of patient engagement and machine learning tools into care delivery networks, and research alliances that connect hub-and-spoke hospitals with larger academic partners (Fig3).
The authors’ experience also suggests that the clinical approach of the physicians on a rural pandemic response team matters. A small team of generalist physicians can in a crisis provide a level of care comparable to that found in academic centers. As the rural subspecialty gap is likely to remain severe30-32, efforts to develop both specialists and subspecialists with a generalist approach within their respective fields will be essential.
To date, the SLH inpatient COVID-19 treatment team has now managed over 500 hospitalized COVID-19 patients and has participated in a number of high profile COVID-19 trials (including ongoing participation in the Operation Warp Speed trials ACTIV-1 and ACTIV-2, which are the immune modulators and outpatient monoclonal antibodies arms, respectively, of the larger Accelerating COVID-19 Therapeutic Interventions and Vaccines initiative through the US government). As a result of this experience, there has recently also been increasing demand for more information about the team’s approach to crafting a local COVID-19 response system33-36. This invites guarded optimism that a tide may be turning and that long-ignored resource disparities and care gaps in rural communities may finally start to get the attention they deserve.
The COVID-19 pandemic has given fresh exposure to a number of longstanding health and healthcare disparities in the USA37,38. One of these healthcare disparities is that of rural Americans and the often under-resourced health systems that serve them. Based upon the authors’ experience over what have now been several phases of COVID-19 in this region, colleagues in academia and rural health policymakers would do well to consider the development of regional hub-and-spoke models for rural settings, to invest in specialty and subspecialty training that is targeted to rural areas, to invest in regional and national clinical rural research networks and to continue to foster strong working relationships between rural physicians and regional academic centers. The development of strong clinical and research networks will help equip rural areas to respond not only to current and future pandemics, but more broadly to any new care delivery challenges that they face. It is hoped that this reported experience can help guide further conversation and the development of further guidelines for treating both COVID-19 and other pandemics and events in rural areas in the future.
References
You might also be interested in:
2016 - Can a 'rural day' make a difference to GP shortage across rural Germany?




