Introduction
Living in a rural community exacerbates the risk of Alzheimer’s disease and related dementias (ADRD) as rural residents face higher incidence of modifiable ADRD risk factors1, are less likely to receive an accurate and timely ADRD diagnosis1, and face significant barriers accessing ADRD caregiving services, which leads to suboptimal care2,3. As a result, rural people living with ADRD rely more on informal care networks4,5, use resource-limited local emergency department services for care over memory and aging services6,7, and spend more time in nursing homes and less time in the community8,9. Therefore, early recognition of ADRD risk and preventative medicine approaches to ADRD are particularly important for persons considered at risk and living in a rural setting.
Subjective cognitive decline (SCD), the subjective experience of worsening memory or cognitive function, is one of the earliest noticeable symptoms of ADRD10. Growing evidence suggests that a significant proportion of those with SCD are subsequently found to develop mild cognitive impairment and ADRD11-13, albeit with a relatively low cumulative conversion rate of 22% and 7% respectively14. This preclinical SCD state offers a therapeutic window where interventions have strong potential to prevent or delay the progression to the ADRD state11,15-18. Out of all the modifiable risk factors for ADRD, physical inactivity is the strongest as it probably contributes to approximately 21% (>1.1 million) of ADRD cases19. Therefore, it is no surprise that exercise is linked to a 30–40% risk reduction for ADRD20-25. However, despite empirical evidence suggesting favorable effects of aerobic exercise (AEx) on ADRD risk, less than 20% of middle-aged and older adults adhere to the current physical activity and aerobic exercise guidelines26, a problem exacerbated in rural areas27.
The top two reported barriers to participation in behavioral, preventative medicine therapies, such as exercise training, include burdens of travel or time commitment28,29. The former is especially relevant in rural-dwelling locations. In addition, rural-dwelling residents report a lack of exercise facilities and other resources as well as social support as major contributors to their self-reported physical inactivity30. Recently, advancements in technology have allowed for home-based telerehabilitation interventions such as exercise training to be utilized as a supplement to or replacement for conventional exercise training programs31-36. Individuals in these home-based exercise programs are able to do so under varying levels of supervision from trained professionals, with some programs having direct supervision (ie synchronous), and others having little to no supervision at all (ie asynchronous). Although telerehabilitation is a growing form of therapy, it has yet to be studied in rural-living residents or in persons at risk for ADRD.
The primary aim of this pilot was to test the feasibility and safety of a synchronous, remotely delivered, AEx program that targeted known barriers to exercise participation in rural-dwelling residents with SCD. We hypothesized that the synchronous, remotely delivered (telerehabilitation) AEx program would not impede a participant’s ability to achieve targets of moderate intensity as outlined by the American College of Sports Medicine37. Specifically, we hypothesized that session attendance would exceed 80% and intensity targets would be achieved in at least 80% of attended sessions. Second, we hypothesized that the AEx telerehabilitation program would be safely conducted, as indicated by absence of adverse events.
Methods
Overview and structure of the rural research team
This pilot research was conducted in collaboration with the Memory Keepers Medical Discovery Team, which employs a community-based participatory research model and utilizes a community advisory group, community-based researchers (CBRs), and a rural community engagement specialist to ensure research studies are aligned with rural community needs and to facilitate the recruitment of participants living in rural northern Minnesota. This applied collaborative approach enables community residents to more actively participate in the full spectrum of research, with a goal of influencing change in community health, systems, programs or policies. Collaborations between local Area Agencies on Aging and other local health organizations are made through existing community advisory group (CAG) members and are used to help with recruitment, inform research design, and share findings.
A six-member Rural Research Advisory Group meets biannually and helps inform the Memory Keepers Medical Discovery Team’s strategic direction for the research. It comprises local, national and international experts and includes the directors of Area Agencies on Aging in northern Minnesota. A 12-member CAG consisting of members who live and work in the rural northern Minnesota meets quarterly. Group members consist of professionals with regional knowledge or expertise of dementia care and services, and individuals who have an interest in older adults or have been directly affected by dementia, including informal caregivers. A person with subjective/early cognitive impairment also sits on the CAG. The CAG advises on both research practice and process as well as community outreach and engagement. The CBRs reside in rural Minnesota and have personal and professional networks with health and civil organizations across the region.
Research design
The Minnesota Rehabilitation Intervention for Dementia Evasion for rural residents (MN RIDE) pilot study employed a single group, pretest–post-test design to assess the feasibility and safety of a synchronous, remotely supervised AEx telerehabilitation program conducted in rural Minnesota communities.
Participants
To be enrolled in the MN RIDE study, participants had to be English-speaking middle-aged or older adults (>45 years) with SCD (indicated by answering yes to both, ‘Do you perceive memory or cognitive difficulties?’ and ‘In the last two years, has your cognition or memory declined?’). In addition, persons were required to identify as rural living or have a mailing address classified as rural based on Rural Health Information Hub38. Persons were excluded if they presented with evidence of objective cognitive decline (a score of <26 on the Telephone Instrument for Cognitive Status39), or diagnosis of mild cognitive impairment or dementia. In addition, persons who had evidence that chemical dependency, neurological condition, or an uncontrolled or a major psychiatric disorder were the likely cause of SCD were excluded. Lastly, persons unable to cycle or with an American College of Sports Medicine exercise contraindication37, were excluded.
Research setting and overview
All screenings, outcome assessments, and interventions occurred within the participants’ place of residence as the MN RIDE project was designed to attenuate barriers to rural exercise participation, including travel to facilities. Recruitment was achieved through multiple strategies, including recruitment into the Center for Community Engaged Rural Dementia and Alzheimer’s Research, research registry and project-specific recruitment brochures and pamphlets with a linked QR code, which directed participants to the study’s Research Electronic Data Capture (REDCap) (https://www.project-redcap.org) recruitment page40,41, where initial screening ensued (S1 phase). Recruitment flyers and pamphlets were distributed through CBRs’ personal and professional contacts, and shared with the local Area Agencies on Aging and Minnesota Senior LinkAge Line, volunteers, community-based service organizations, fitness centers, clinic wellness programs, and public health departments across rural communities in northern Minnesota. Most participants were identified and recruited through face-to-face interactions with the CBRs. This allowed the Memory Keepers Medical Discovery Team to fill recruitment quota more efficiently than more traditional methods of using a registry or leaving recruitment flyers in public spaces. Community-based strategies tailored to the unique regional and cultural context of rural residents’ help reduce feelings of mistrust towards medical research, a barrier to the recruitment of rural populations42.
Upon review of the S1 REDCap survey, a CBR completed the S1 screen by phone, which included administration of the Telephone Instrument for Cognitive Status to gauge objective cognitive status. Consenting was carried out remotely at the second screening (S2) visit via electronic informed consent and Zoom, as electronic informed consent was administered through REDCap, a web-based, data collection system that has been validated as compliant with the US Food and Drug Administration Title 21 Code of Federal Regulaton Part 1140,41. Upon completion of the virtual electronic informed consent, participants were emailed links for completing REDCap surveys that further assessed SCD status, affective conditions, and health and medical history with the purpose of screening for exercise contraindications and exclusionary criteria specific to likely cause of SCD. After completion of the virtual S2 visit and REDCap surveys, study staff faxed each participant’s primary care provider, which served to inform them about their study participation, and requested their written clearance validating approval of study participation. Primary care provider written clearance was sought to ensure that: no exercise contraindications were present, and causes of SCD were not attributed to major psychological disorder, metabolic disorder, or induced by medication or chemical dependency. Upon receipt of the primary care provider clearance (screening 3 (S3) phase), study staff purchased a recumbent cycle ergometer, automated blood pressure monitor and pulse oximeter, which were directly shipped to the participant’s place of residence. In the event that a participant was without a computer/laptop or smart device capable of Zoom, they were loaned a study iPad. As incentive for participating in the MN RIDE study, all study participants were able to keep the recumbent cycle ergometer upon completion of the study.
At the baseline assessment, a CBR traveled to the participant’s place of residence to complete the physical function assessment and help provide troubleshooting for Zoom and other equipment use in preparation of the synchronous telerehabilitation AEx program. CBRs traveled to the participants’ places of residence for the baseline visit and again for the 12-week (follow-up) assessments. Participants were concentrated within 12–129 km (8–80 miles) of CBRs’ homes. The study area of northern Minnesota is a large geographic area with low population density, stretching roughly 386 km (240 miles) east to west and north to south and includes 26 counties. In this geographic area, which has a population of 627 812, census data indicate that 61.0% live in rural areas and 44.7% are aged 45 years or older.
Immediately following the in-person baseline assessments, the first AEx session was conducted remotely supervised by the study interventionist. The CBR who conducted the in-person baseline assessments remained present during this first session for troubleshooting if delivery or equipment issues arose. During this session, the CBR was able to directly evaluate objective and subjective indicators of tolerance to AEx and communicate this to the study interventionist (exercise therapist) who was supervising the session using a synchronous audiovisual telerehabilitation format (Zoom). All remaining exercise sessions (2–36) were then facilitated by the exercise therapist over Zoom, where all participants were required to have their audio and video turned on so the exercise therapist could fully evaluate the session and communicate with participants. Following the completion of each session, the exercise therapist summarized the results of the session (time spent in RPE and HRmax target zones) with the participant for positive reinforcement and scheduled the day and time of the next session in an attempt to promote greater attendance. No modifications were made to the delivery or format of the intervention over the course of the pilot study.
MN RIDE intervention
The synchronous, remotely supervised AEx intervention was conducted with recumbent stationary cycles (Exerpeutic 900SL; Industry, CA) at moderate intensity, at a frequency of three times per week. Participants were able to choose three separate 1-hour blocks (Monday–Saturday, 7am to 6pm) to schedule their exercise session with the therapist that aligned best with their schedule. A routine was encouraged, but not required. The predominant exercise intensity measure used to guide exercise prescription was the Borg Category Ratio-15 Rating of Perceived Exertion Scale43. Secondary metrics of exercise intensity evaluated included the percentage of age-predicted maximum heart rate (HRmax) (in persons without cardiovascular disease or heart rate altering medications)37. Cycling was progressed from RPE 11–12 for 30–35 minutes in session 1, and was alternatively increased by 1-point on Borg or 5-minute increments as tolerated up to RPE 12–14 for 50 minutes a session over time (by session 18). Target heart rate zones for participants represented HRmax 64–76%37. In all sessions, heart rate was continuously monitored with a heart rate monitor located on handgrips on the cycle ergometer as well as a pulse oximeter. Each session included a 5-minute warm-up and cool-down in addition to the prescribed exercise duration.
Main outcomes and measures
Feasibility of synchronous telerehabilitation: Attendance was recorded and total number of sessions attended/36 possible sessions over 12 weeks was quantified to give one quantitative metric of intervention feasibility. Second, in each session, RPE and heart rate that were recorded over each 5-minute interval of exercise were averaged for each participant. Cumulated averages for RPE and HRmax were quantified and compared to published American College of Sports Medicine exercise prescription guidelines for moderate intensity37. Using both an objective and a subjective metric for intensity provided a comprehensive indicator of feasibility of an AEx telerehabilitation to achieve moderate intensity targets, as recommended in current exercise prescription guidelines37. Participant adherence (attendance) was considered acceptable and optimal at thresholds of 60% and ≥80% respectively. Likewise, if 60% of attended sessions achieved RPE or HR targets, this was considered an acceptable threshold for intensity adherence, while ≥80% represented an optimal threshold.
Safety/adverse events (AE): Data regarding non-study, study-related, and type of AEs were tracked throughout the study and used as a surrogate of safety-related outcomes. Non-study-related AEs were categorized as either ‘clearly not related to the study’ or ‘doubtfully related to the study’. Study-related AEs were categorized as event ‘possibly’, ‘likely’, or ‘clearly’ related to the study. Further, type of AE (cardiac, limb, musculoskeletal, metabolic, other) and incidence, were recorded. Lastly, the severity of AE was categorized as minor, moderate, or serious. All AEs were assessed and graded by study investigators. Study-related AEs per number of training hours were calculated for each group.
Qualitative feedback from MN RIDE participants: At the end of the MN RIDE program, participants were administered an exit interview composed of 11 open-ended questions and two closed-ended questions. Questions were designed to evaluate the satisfaction of the MN RIDE program, fine-tune the intervention for future, and gauge the potential sustainability of the MN RIDE program in rural communities.
Statistical analysis
Means and standard deviations were quantified for feasibility measures (ie adherence (attendance) and intensity compliance). All data were analyzed using SPSS v28.0 (IBM Corp; https://www.ibm.com/spss).
Ethics approval
This study was in compliance with the Helsinki Declaration. Ethics approval was obtained from the University of Minnesota Institutional Review Board (STUDY00012097).
Results
Participant characteristics
The enrolment goal of the MN RIDE study was 10 participants, with active recruitment taking place during March–April 2022. Twenty-one people expressed interest in the MN RIDE study by scanning QR codes on recruitment materials (ie study brochures). Potential participants completed the following screening phases S1 (n=10), S2 (n=10), and S3 (n=9). The nine participants who met eligibility criteria and were enrolled (Fig1). The average age of the study sample (n=9) was 57.44±7.16 years (average age of SCD onset 53.44±7.47 years) with 14.00±5.57 years of education and 88.9% female (Table 1).
Table 1: Descriptive characteristics of MN RIDE study participants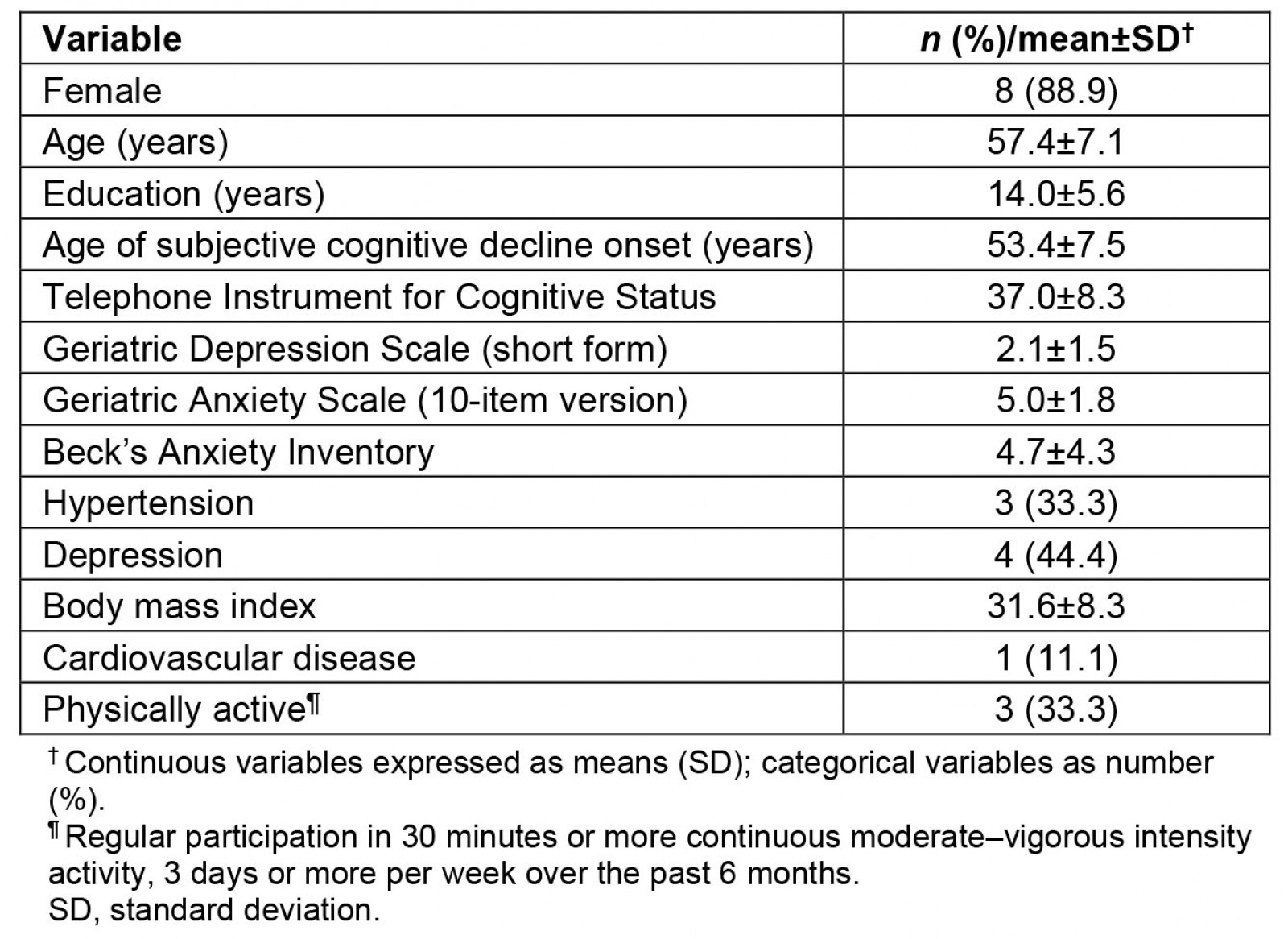
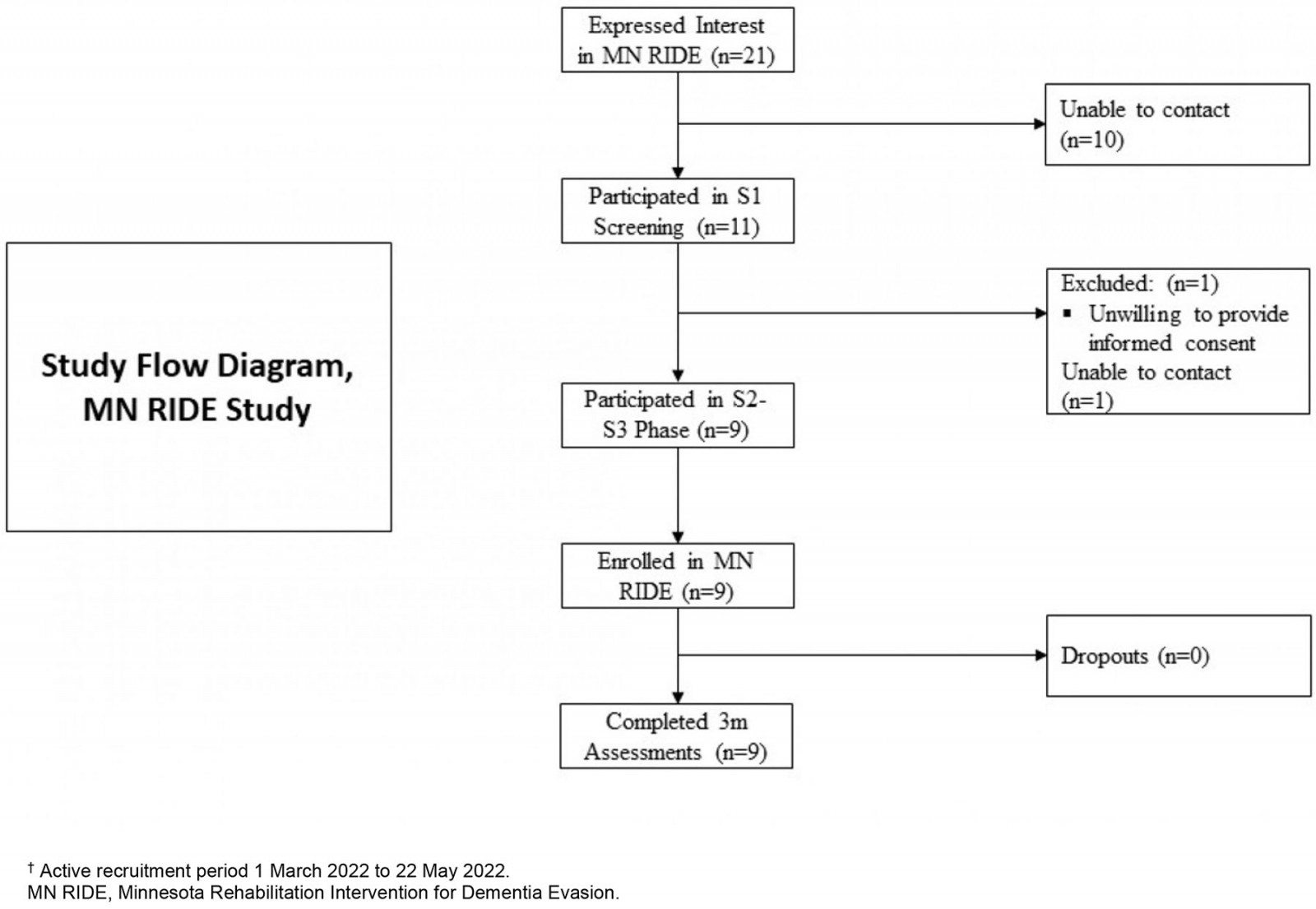 Figure 1: Flow diagram for the MN RIDE study.†
Figure 1: Flow diagram for the MN RIDE study.†
Feasibility and safety
All patients completed the study, resulting in a dropout rate of 0%. All participants had internet access and a smart device to complete the synchronous audiovisual delivery of the MN RIDE program. There were no reports of difficulties with managing any of the electronic devices or equipment required for the successful completion of study activities. Overall, participants attended on average 85% of possible sessions over the course of the 12-week AEx telerehabilitation program. Of the total 276 training sessions completed, 214 (78.0%) had average RPE that reached the target intensity cut-off of 13 and 237 (85.8%) had average heart rates that met the target minimal intensity threshold of 64% HRmax. Collectively, over the 276 training sessions, the average RPE was 13.2±0.5, while the HRmax was 72.0±7.9% (Table 2). On average, participants exercised 21.6 hours (82.7% of the total prescribed exercise duration (26.1 hours) over 12 weeks). Overall, there were no study-related (likely caused by intervention) AEs in 215 training hours that occurred and no in-person interventions or troubleshooting was required by study interventionists outside of the first in-person exercise session.
Table 2: Feasibility and safety metrics of the MN RIDE study. RPE and HRmax reflect averages over the course of each attended exercise session. Adverse events reflect any adverse event that possibly or likely attributed to the intervention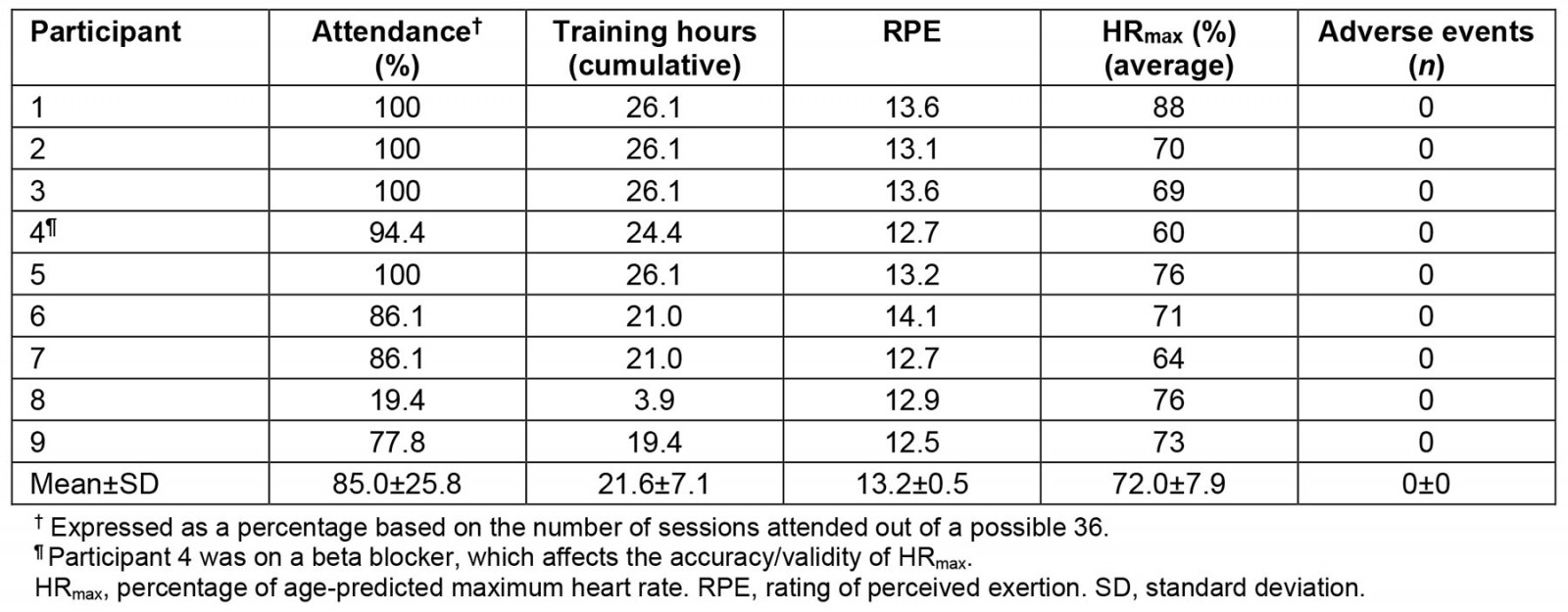
Participant satisfaction
Findings from the open-ended questions pertaining to the satisfaction and sustainability of MN RIDE are presented in Table 3. Eight of the nine participants (88.9%) reported that they would recommend the MN RIDE program to friends or family.
Table 3: Summary of MN RIDE exit interviews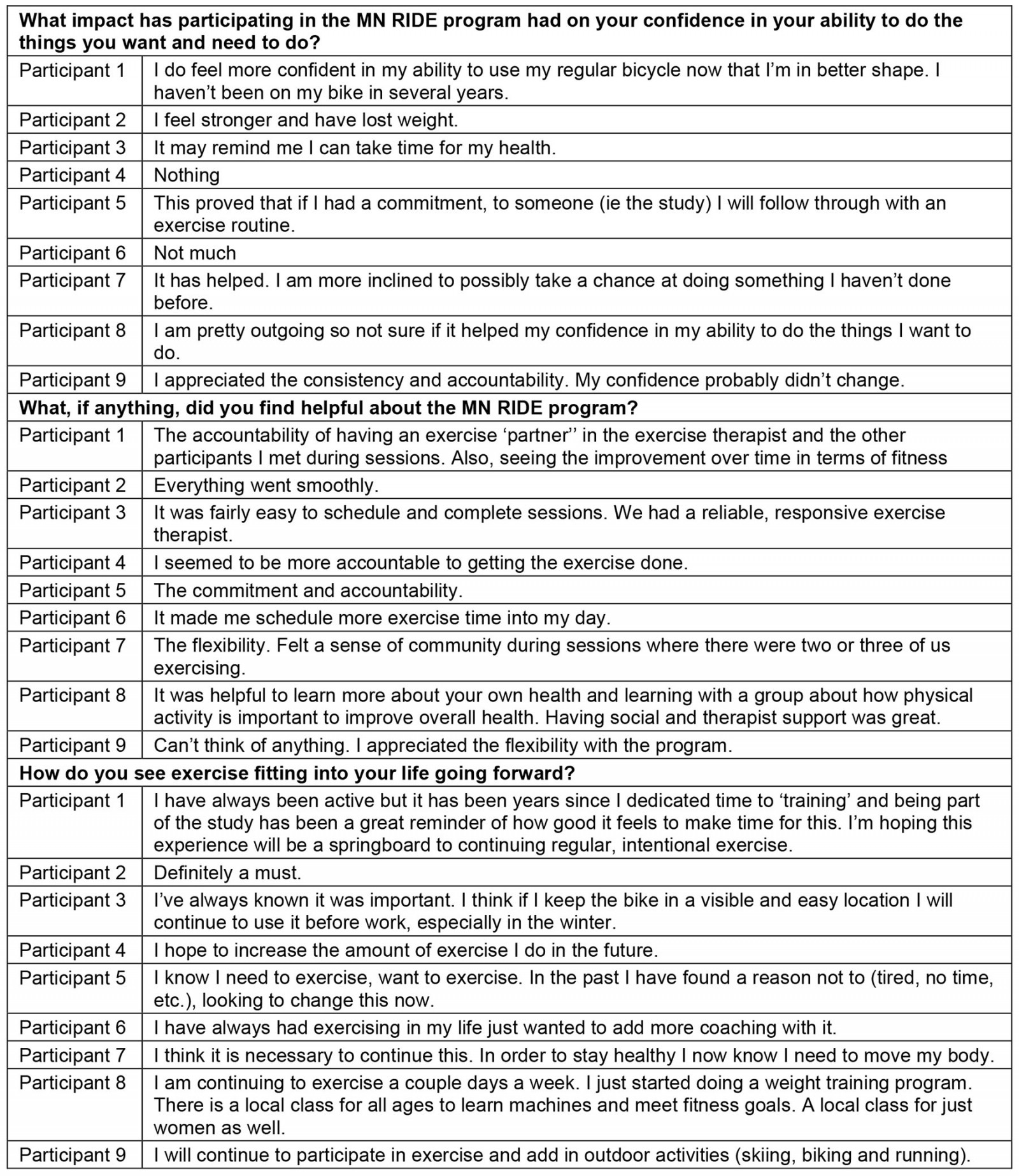
Discussion
Adherence to exercise has been operationally defined as ‘the extent to which an individual corresponds with the quantity and quality of exercise, as prescribed by their healthcare professional’44. Strategies that are postulated to enhance exercise adherence include supervision, education, personalized exercise prescription, social support, and self-monitoring45. Access barriers to exercise participation in supervised, facility-based exercise programs include limited program availability, poor or cold weather, costs, transport restrictions, and inconvenient program scheduling, which are significantly amplified in rural communities46. In this sample, six out of nine participants communicated barriers that have affected their ability to exercise in the past (time (n=3), lack of equipment and facilities (n=2), and family/work obligations (n=1)). The MN RIDE program provided an exercise program that incorporated aspects of known facilitators to exercise participation including direct (virtual) supervision of AEx sessions, personalized AEx prescriptions, and sessions conducted with multiple participants (which is a facilitator of social interaction and accountability). Since the participants were shipped needed exercise equipment, able to participate in AEx sessions from their place of residence, and able to schedule three sessions per week (Monday–Saturday, 7am to 6pm) with the study exercise therapist, the MN RIDE program was also able to combat the traditional access barriers to supervised, facility-based exercise programs listed above.
The ability of the MN RIDE program to successfully address these facilitators and barriers to exercise participation in rural communities probably contributed to the high (85%) session attendance, which supports the study’s first hypothesis that attendance would exceed 80%. Furthermore, in removing one participant (22% attendance) who was in the midst of moving out of the state, the remaining eight participants had an average of 93.6% attendance (range 78–100%) with four having perfect attendance. Overall, the 85% session attendance is superior to what is classically reported in 12-week, 36-session supervised facility-based exercise programs that are predominantly found in urban settings, including cardiac rehabilitation (68.9%)47 and supervised exercise therapy for peripheral artery disease (44.4%)48.
Pertaining to the quality of exercise provided by MN RIDE, moderate–vigorous AEx was prescribed based on the evidence suggesting its favorable effects on the management of ADRD risk factors (ie hypertension, diabetes mellitus, obesity, hyperlipidemia, depression, anxiety)49-52. Again, participants exercised successfully at moderate intensity (72% HRmax (range 64–88%), and RPE 13.2 (range 12.5–14.1)), at 82% of the prescribed exercise duration. These findings suggest the potential of the MN RIDE intervention to also deliver quality AEx and supported the study’s second hypothesis. Collectively, the high adherence to attendance, session duration, and intensity are important to highlight in the context of exercise dose (ie the product of three discrete exercise prescription variables: duration, frequency, and intensity), given the dose–response relationships with certain ADRD risk factors, including hypertension49, diabetes mellitus53, depression54, and anxiety55.
Safety is a major concern with exercise telerehabilitation programs such as in the format of the synchronous, audiovisual delivery model inherent to the MN RIDE program. This concern is predominantly because of the limited possibility of direct intervention by the exercise therapist in the case of AEs. Findings regarding AEs present in the MN RIDE study are in line with the results of previous studies underlying the safety of exercise telerehabilitation56. The MN RIDE program promoted participant safety through the implementation of a thorough screening process where participants’ health histories were evaluated by a clinical exercise physiologist for exercise contraindications, and participants’ primary care providers provided written clearance for participation in the telerehabilitation program. Additionally, in each exercise session, vitals were assessed through the use of pulse oximetry and blood pressure monitors and monitored by the supervising exercise therapist.
The major strength of this study is the design and infrastructure of the MN RIDE intervention. The Memory Keepers Medical Discovery Team provided a strong foundation to launch this pilot program. given their experience in conducting rural health research in northern Minnesota, and was important for successful implementation of this pilot study. Second, although a cost comparison can only be estimated, and a thorough analysis is outside the scope of this article, we can state the telerehabilitation model is probably more cost effective than traditional in-person delivery models (ie gym membership + personal trainer). The overall estimated cost for one participant to complete all 36 sessions of the program was approximately $1001 (cycle ergometer US$229 (A$354), pulse oximeter US$25 (A$39), automated blood pressure monitor US$27 (A$42), university-employed exercise therapist US$720 (A$1113) (US$20(A$31)/session for 36 sessions)). In comparison, an in-person delivery model in this area (urban fitness facility in northern Minnesota) for 3 months would cost approximately US$1860 (A$2876) (sign-up fee + 3-month adult membership fee US$260 (A$402); 36 sessions with personal trainer US$1600 (A$2472)). Cost savings could also be implemented when employing an intervention that uses a 1 : 2 or 1 : 3 exercise therapist to participant ratio. However, it should also be noted that all participants had a computer or smart device and internet for synchronous audiovisual communication with study therapists. In northern Minnesota, it is estimated that 87.5% of households have some type of broadband internet access57. A need to provide internet capacity (ie hot spot) or smart device would also increase the cost of the intervention. Although many barriers to physical activity/exercise in rural communities were addressed in the design of the MN RIDE intervention, cost and internet access may still be considered barriers/challenges for future studies or implementation of similar interventions in rural communities.
In addition to the strengths of the study, there are weaknesses that must also be discussed. We acknowledge that this exploratory study suffers from limitations due to the non-controlled design and the small sample size. Regarding the number of subjects, this was a pilot study; thus a precise sample size calculation was not carried out as the research team’s primary focus was to gain insight primarily on process implementation and feasibility of rural-focused synchronous AEx telerehabilitation delivered through a CBR structure. The generalizability of the findings may be affected because it was 100% White Caucasian, with 77.9% reporting ‘some college’ regarding educational achievement (in northern Minnesota 23% report ‘some college’57), and a majority female representation. However, we believe the latter could be interpreted as a strength given the underrepresentation of females in clinical research and exercise intervention studies58-60. Finally, the inability to gauge the effectiveness of the MN RIDE intervention on exercise and non-exercise physical activity in the short and long term (through wearable technologies) make the true assessment of sustainability impossible to fully determine.
Conclusion
Collectively, we believe that the design of the MN RIDE intervention promoted the delivery of safe and quality AEx, which in turn promoted high attendance and 0 dropouts. Our findings add to the growing evidence that synchronous audiovisual telerehabilitation is a safe and feasible model for delivery of exercise therapies in persons who experience barriers to regular exercise participation. This pilot study is the first to provide evidence of preliminary feasibility of synchronous audiovisual telerehabilitation programs delivered to rural residents at risk for ADRD. Thus, exercise telerehabilitation programs that focus on AEx could be viable and useful tools to overcome situations with limited access to healthcare services such as in rural communities. Further controlled studies with greater sample size could help further expand our results.
Funding
This research was supported by a University of Minnesota Medical School’s Academic Investment Research Program award to the Center for Community Engaged Rural Dementia and Alzheimer’s Research (KJ and WW).
Conflict of interest
The authors report no competing interests in relation to this article.
References
You might also be interested in:
2020 - Community acquired pneumonia: a cost-of-illness analysis in Greece

