Introduction
In the US, the ‘fourth wave’ of the opioid epidemic, marked by the simultaneous consumption of N-methylamphetamine (methamphetamine) and opioids, has disproportionally impacted rural communities, particularly in western states1,2. A recent cross-sectional study conducted between 2018 and 2020 in 10 states and 65 rural counties of the US found that 75% of opioid users reported past 30-day methamphetamine use3, contributing to the steeper increase in opioid overdose rates in rural areas when compared to urban areas seen in the past two decades (325% v 198%)4.
Independent of geographic location, when compared to opioid users alone, those co-using opioids and methamphetamine are more likely to be homeless, have comorbid severe psychiatric illness, and be infected by sexually transmissible infections such as HIV and hepatitis C5-11. In addition, overdose mortality rates among people who co-use opioids and methamphetamine are higher than those seen for people who use opioids or methamphetamine alone, highlighting the risk of using both substances together1,12. In 2021, more than 60% of all methamphetamine overdoses also involved opioids13,14.
Medications for opioid use disorder (MOUD), such as methadone and buprenorphine, have been shown to be effective at preventing opioid withdrawal, use, and overdose15-17. However, research suggests that, for individuals receiving MOUD, concomitant methamphetamine use negatively impacts treatment retention and opioid abstinence over time18-22. Additionally, among patients seeking substance use treatment, patients with concomitant methamphetamine and opioid use are less likely to receive MOUD compared to those using opioids only23. This is particularly concerning given that 61% of opioid users receiving MOUD in rural settings have reported co-using methamphetamine3.
As opioid and methamphetamine co-use continues to rise in rural counties of the US, the need to evaluate the clinical profile and the prognostic effects of methamphetamine use among individuals initiating MOUD treatment in rural community treatment settings is paramount to understand the needs of these vulnerable, underserved, and understudied populations24,25. Within this context, the objective of this retrospective cohort study was to determine if initiating MOUD treatment with a positive methamphetamine urinalysis (UA) result is associated with a worse clinical profile at intake and worse treatment outcomes (opioid use over time, methamphetamine use over time and retention to treatment) when compared to initiating treatment with a negative methamphetamine UA result. Based on prior evidence, we hypothesize that patients initiating MOUD treatment with a positive methamphetamine UA result would have a more severe clinical profile at intake and would experience worse treatment outcomes when compared to those initiating MOUD treatment with a negative methamphetamine UA result19,21,22,26.
Methods
Design and study location
This 1-year retrospective cohort study was conducted in collaboration with four Oregon Recovery and Treatment Centers (ORTCs), all located in rural Oregon in cities with populations between 15,000 and 100,000 people. (To protect patient identities, municipalities of treatment sites are not disclosed.) ORTCs are specialized clinics that treat patients with opioid use disorder through evidence-based interventions such as MOUD (eg methadone and buprenorphine), individual and group counseling, contingency management, and group therapy based on the Matrix Model to assist opioid users in different stages of recovery27. Of the patient population at ORTC, 95% of individuals are covered by Medicare or Medicaid, with 89% of those being covered by Medicaid.
Sample
Data used for this study were extracted from the electronic medical records of patients from the four ORTC clinics whose intake assessments occurred between 1 January and 31 December 2019. All individuals initiating MOUD treatment at any of the four ORTC clinics during this period were included in this study (n=554).
Assessments
Intake assessments included recording of sociodemographic data and DSM-5 diagnoses for opioid use disorder, depression, anxiety, and post-traumatic stress disorder28. Information on sexually transmitted diseases, such as hepatitis and HIV, was determined by self-report. Patients also provided UA samples for methamphetamine, opioids, cocaine, THC (tetrahydrocannabinol), and benzodiazepines at treatment intake and once per month, for a 1-year period. Notably, providers used UA sample results exclusively, and without judgement, to assist them to determine the best treatment for their patients.
Outcomes
Our primary outcomes were opioid and methamphetamine use, and treatment retention over a 1-year period. Objective verification of opioid and methamphetamine use was determined using UA results collected once per month. Treatment retention was determined by considering the number of days elapsed from treatment intake to treatment dropout.
Statistics
Intake demographics and clinical profile were compared by methamphetamine UA results collected at intake using ANOVA (analysis of variance) for continuous variables and χ2 tests for categorical variables.
To evaluate the impact of methamphetamine use at treatment intake on methamphetamine use over time, we used a Generalized Estimating Equations (GEE) approach for longitudinal binary outcomes. For this model, methamphetamine UA result at intake assessment (or visit 0) was assigned as our primary predictor of interest. Sex, age, time, and the methamphetamine UA result at treatment intake over time, were included as adjustments, together with employment status, as this variable was unevenly distributed among groups (Table 1). Thus, the longitudinal assessment data (months 1–11) for participants’ methamphetamine UA results constituted the outcome measure analyzed in this model. The assessment data were coded as either ‘1’ for a negative methamphetamine UA result or ‘0’ for a positive methamphetamine UA result. Due to the nature of the outcomes’ correlation structure, a binary logistic model with an autoregressive structure of order 1 was used.
To evaluate the impact of methamphetamine use at intake on opioid use over time, we created a similar GEE model using each participant’s 11 opioid UA results as the longitudinal binary outcomes. We intended to include the opioid UA result of intake as an adjustment; however, after detecting a high collinearity with our main predictor (methamphetamine UA result at treatment intake), we decided to exclude it from our model.
Lastly, we conducted a Kaplan–Meier survival analysis, assigning the methamphetamine UA result at treatment intake as the main predictor and the time elapsed from treatment intake to discharge as the outcome. All statistical analyses were performed using R v4.0.2 (R Foundation for Statistical Computing; https://www.r-project.org), with the significance level set at 0.05.
Table 1: Sociodemographic and clinical profile by methamphetamine urinalysis results at treatment intake in rural Oregon, January–December 2019
| Characteristic |
Positive meth UA (n=277, 50%) |
Negative meth UA (n=277, 50%) |
p-value | |
|---|---|---|---|---|
| Clinic, n (%) | Site A | 43 (15.5) | 57 (20.6) | 0.223 |
| Site B |
90 (32.5) |
99 (35.7) | ||
| Site C |
87 (31.4) |
73 (26.4) | ||
| Site D |
57 (20.6) |
48 (17.3) | ||
| Age, mean±SD |
37.2±10.9 |
36.3±10.7 | 0.338 | |
| Sex, n (%) | Female | 132 (47.7) | 112 (40.4) | 0.218 |
| Male |
142 (51.3) |
160 (57.8) | ||
| Transgender |
1 (0.4) |
2 (0.7) | ||
| Race, n (%) | White | 251 (90.6) | 245 (88.4) | 0.554 |
| Native American |
11 (4.0) |
10 (3.6) | ||
| Hispanic |
12 (4.3) |
12 (4.3) | ||
| African-American |
1 (0.4) |
4 (1.4) | ||
| Asian |
0 (0.0) |
1 (0.4) | ||
| Alaska Native |
0 (0.0) |
1 (0.4) | ||
| Other |
0 (0.0) |
1 (0.4) | ||
| Employed, n (%) |
101 (36.5) |
78 (28.2) | 0.048* | |
| Homeless, n (%) |
17 (6.1) |
13 (4.7) | 0.580 | |
| Legal issues, n (%) |
68 (24.5) |
77 (27.8) | 0.469 | |
| Opioid use disorder diagnosis, n (%) | Moderate | 21 (7.6) | 23 (8.3) | 0.855 |
| Severe |
254 (91.7) |
250 (90.3) | ||
| Positive opioid UA, n (%) |
245 (88.4) |
127 (45.8) | <0.001*** | |
| Positive cocaine UA, n (%) |
6 (2.2) |
9 (3.2) | 0.591 | |
| Positive THC UA, n (%) |
120 (43.3) |
108 (39.0) | 0.360 | |
| Positive benzodiazepine UA, n (%) |
23 (8.3) |
24 (8.7) | 1.000 | |
| Depression, n (%) |
62 (22.4) |
76 (27.4) | 0.200 | |
| Anxiety, n (%) |
95 (34.3) |
92 (33.2) | 0.881 | |
| PTSD, n (%) |
46 (16.6) |
42 (15.2) | 0.713 | |
| Hepatitis, n (%) |
44 (15.9) |
47 (17.0) | 0.818 | |
| HIV, n (%) |
0 (0.0) |
1 (0.4) | 0.999 | |
*p<0.05, **p<0.01, ***p<0.001
meth, methamphetamine. PTSD, post-traumatic stress disorder. SD, standard deviation, THC, tetrahydrocannabinol. UA, urinalysis.
Approach for missing data
In light of the high dropout rate observed in this study (Fig1) – and our desire to comply with the intention-to-treat paradigm while acknowledging the difficulties in determining the best approach for missing data in substance use disorder treatment trials – our research group opted to analyze missing data for our GEE analyses in three ways. Missing data were considered as either missing (ie default), positive (worst-case scenario), or through multiple imputations carried out using sequential regression imputation (multiple imputation). Multiple imputations were carried out based on Rubin’s rules for combining multiple imputation analyses, and 70 datasets were imputed and pooled to produce aggregate estimates29. No imputation was needed to achieve the intention-to-treat paradigm for the treatment retention outcome analysis.
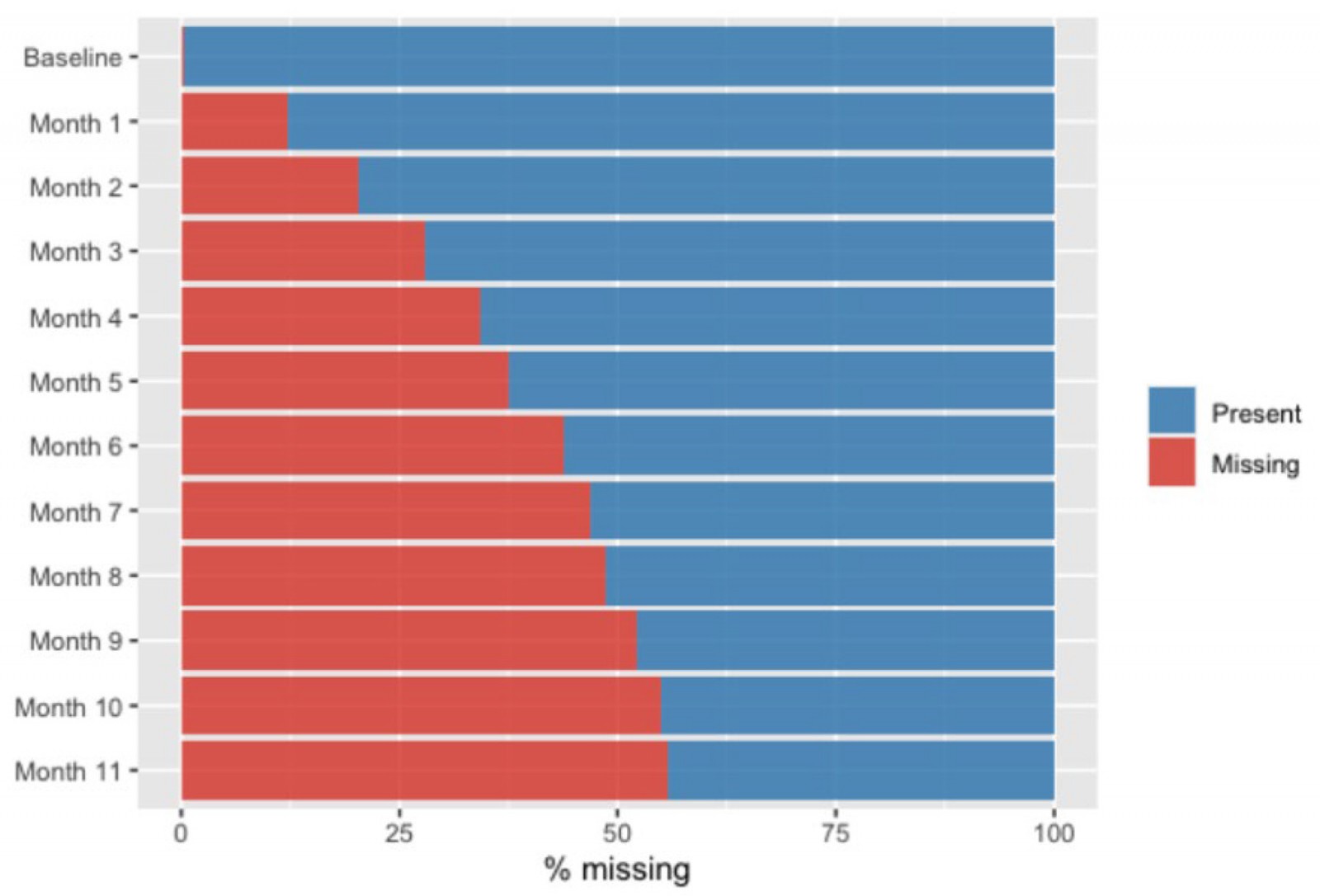 Figure 1: Prevalence of missing urinalysis data over time for treatment participants in rural Oregon, January–December 2019.
Figure 1: Prevalence of missing urinalysis data over time for treatment participants in rural Oregon, January–December 2019.
Ethics approval
To comply with the Health Insurance Portability and Accountability Act of 1996, only unidentifiable data was extracted from ORTC electronic medical records. As a result, the Washington State University Internal Review Board determined this study to be exempt from ethics approval.
Results
Intake characteristics
As shown in Table 1, in 2019 a total of 554 patients enrolled at one of these four rural MOUD clinics, of which 277 (50%) had a negative methamphetamine and 277 (50%) had a positive methamphetamine UA result at intake. The sample mostly comprised White individuals (89.5%), half of participants were male (54.5%), and the mean age was 36.8 years (SD 10.8 years). A third were unemployed (32.3%), more than a quarter reported legal problems (26.2%), and 5.4% of participants were currently homeless.
Most participants had a severe opioid use disorder diagnosis (91.0%) while 7.9% had a diagnosis of moderate opioid use disorder. Most patients had a positive opioid UA result at intake (67.1%), and positive UA results for methamphetamine (50%), THC (41.2%), benzodiazepine (8.5%), and cocaine (2.7%). The prevalence of specific psychiatric comorbidities among all patients was highest for anxiety (33.8%), followed by depression (24.9%) and post-traumatic stress disorder (15.9%). Lastly, 16.4% of participants had a known diagnosis of viral hepatitis and 0.2% had a known diagnosis of HIV. When compared to those testing negative for methamphetamine, participants initiating MOUD treatment with a positive methamphetamine UA result were more likely to be unemployed (36.5% vs 28.2%; p=0.048) and to have a positive opioid UA result at intake (88.4% vs 45.8%; p<0.001). No other demographic or clinical feature assessed at intake differed statistically between the two groups.
Methamphetamine use over time
Regardless of how missing data were handled, our GEE analyses consistently showed that those initiating MOUD treatment with a negative methamphetamine UA result were significantly more likely to have a negative methamphetamine UA result over time compared to those initiating MOUD treatment with a positive methamphetamine UA result (Table 2). Odds ratios (ORs) ranged from 1.35 to 1.55, depending on how missing data were analyzed (p<0.22 for default; p<0.087 for worst-case scenario for all). The worst-case-scenario model showed an effect of time (OR=0.92; p<0.01), suggesting that the odds of having a negative methamphetamine UA result decreased over time independently of methamphetamine UA status at intake and all other covariates. There were no other significant associations.
Table 2: Methamphetamine abstinence over time by methamphetamine urinalysis results at treatment intake in rural Oregon, January–December 2019†
| Characteristic | Default | Multiple imputation | Worst-case scenario | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95%CI | p-value | OR | 95%CI | p-value | OR | 95%CI | p-value | |
| Negative meth UA at intake | 1.55 | 1.06–2.25 | 0.022* | 1.52 | 1.05–2.20 | 0.028* | 1.35 | 0.85–1.70 | 0.087 |
| Time | 1.01 | 0.98–1.04 | 0.535 | 1.018 | 0.98–1.06 | 0.350 | 0.92 | 0.91–0.96 | 0.001** |
| Negative meth UA at intake over time | 0.99 | 0.96–1.04 | 0.939 | 1.006 | 0.96–1.05 | 0.801 | 0.99 | 0.96–1.05 | 0.849 |
| Male | 1.03 | 0.74–1.43 | 0.859 | 1.073 | 0.77–1.50 | 0.681 | 1.17 | 0.90–1.58 | 0.627 |
| Transgender | 0.26 | 0.04–1.73 | 0.163 | 0.231 | 0.01–6.16 | 0.414 | 0.03 | 0.004–0.12 | <0.001*** |
| Age | 1.01 | 0.99–1.02 | 0.243 | 1.001 | 0.99–1.02 | 0.927 | 1.01 | 0.99–1.02 | 0.062 |
| Unemployed | 0.93 | 0.65–1.32 | 0.667 | 1.010 | 0.68–1.49 | 0.962 | 0.93 | 0.71–1.31 | 0.565 |
*p<0.05, **p<0.01, ***p<0.001
†Reference levels are positive methamphetamine urinalysis at intake, female, and employed, respectively
CI, confidence interval. meth, methamphetamine. OR, odds ratio, UA, urinalysis.
Opioid use over time
Independent of methamphetamine UA status at intake, time was significantly associated with the odds of a negative opioid UA result over time (Table 3). The odds of having a negative opioid UA result over time increased in the default and multiple imputation models (OR=1.05; p<0.001 and OR=1.053; p<0.021, respectively), but decreased in the worst-case-scenario model (OR=0.94; p<0.001). For all models, no other association achieved statistical significance.
Table 3: Opioid abstinence over time by methamphetamine urinalysis results at treatment intake in rural Oregon, January–December 2019†
| Characteristic | Default | Multiple imputation | Worst-case scenario | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95%CI | p-value | OR | 95%CI | p-value | OR | 95%CI | p-value | |
| Negative meth UA at intake | 1.39 | 0.96–2.02 | 0.084 | 1.303 | 1.05–2.20 | 0.192 | 1.20 | 0.85–1.70 | 0.297 |
| Time | 1.05 | 1.02–1.09 | 0.001*** | 1.053 | 0.98–1.06 | 0.021* | 0.94 | 0.91–0.96 | <0.001*** |
| Negative meth UA at intake over time | 0.99 | 0.95–1.05 | 0.849 | 1.010 | 0.96–1.05 | 0.650 | 1.00 | 0.96–1.05 | 0.832 |
| Male | 1.08 | 0.79–1.47 | 0.627 | 1.172 | 0.77–1.50 | 0.349 | 1.19 | 0.90–1.58 | 0.215 |
| Transgender | 0.03 | 0.01–0.12 | <0.001*** | 0.288 | 0.01–6.16 | 0.372 | 0.02 | 0.004–0.12 | <0.001*** |
| Age | 1.01 | 0.99–1.03 | 0.062 | 1.012 | 0.99–1.02 | 0.085 | 1.01 | 0.99–1.02 | 0.130 |
| Unemployed | 0.91 | 0.65–1.27 | 0.565 | 0.870 | 0.68–1.49 | 0.365 | 0.97 | 0.71–1.31 | 0.829 |
*p<0.05, **p<0.01, ***p<0.001.
†Reference levels are positive methamphetamine urinalysis at intake, female, and employed, respectively.
CI, confidence interval. meth, methamphetamine. OR, odds ratio, UA, urinalysis.
Treatment retention
A total of 96 patients (34.7%) with a positive methamphetamine UA result at intake remained in treatment after 1 year, compared to 107 (37.9%) with a negative methamphetamine UA result at intake. As shown in Figure 2, the Kaplan–Meier survival analysis found no significant statistical effect of methamphetamine UA result at intake on treatment retention (p=0.51).
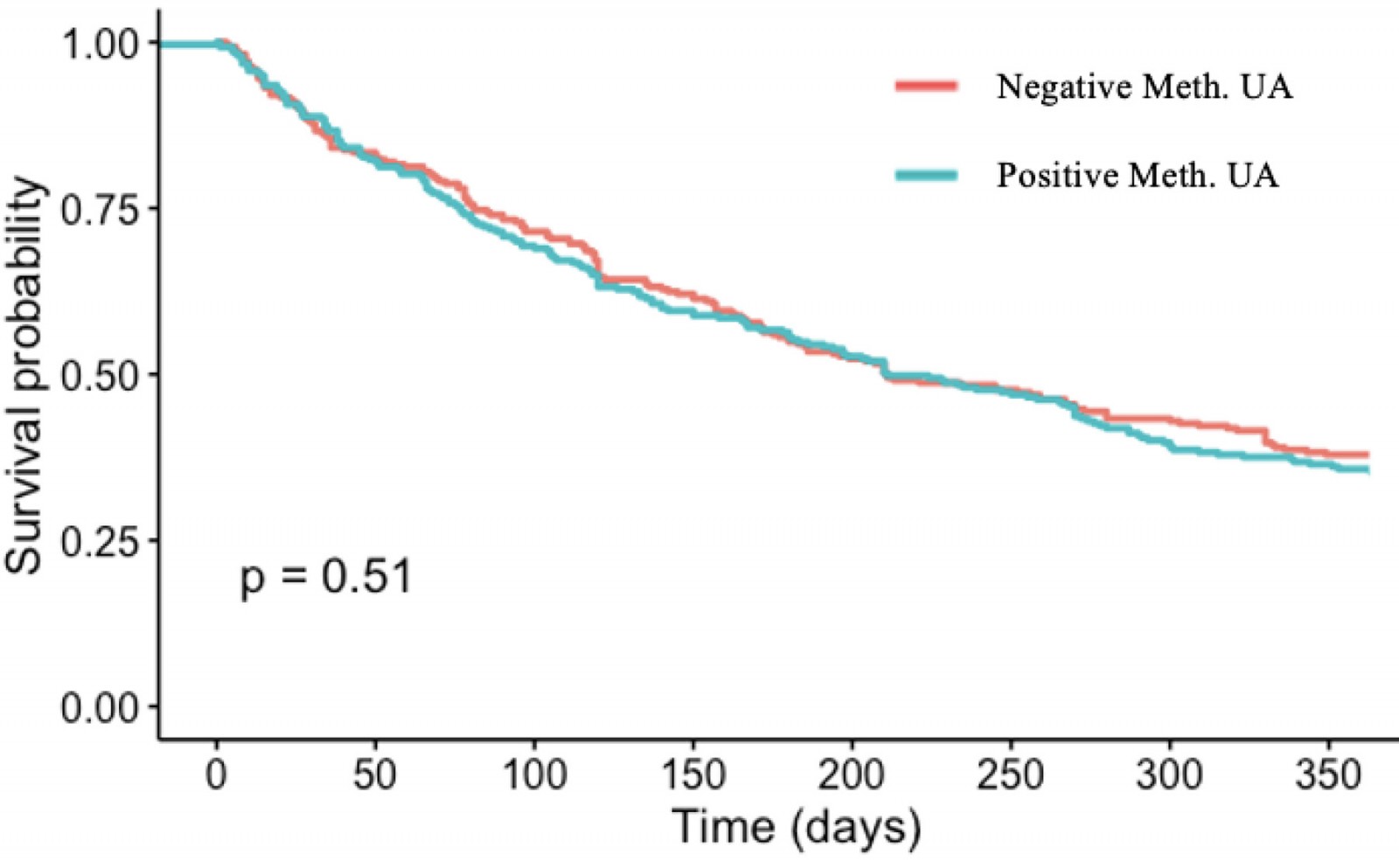 Figure 2: Treatment retention survival analysis – effect of methamphetamine urinalysis result at intake
Figure 2: Treatment retention survival analysis – effect of methamphetamine urinalysis result at intake
Discussion
While the neuromechanisms involving opioid and methamphetamine co-use are not fully understood, it is well established that both opioid and methamphetamine exert reinforcing effects, in part, by increasing dopamine release through the mesolimbic pathway and, eventually, the nucleus accumbens, thus increasing pleasure levels when both drugs are consumed concurrently30,31. For instance, studies have found that simultaneous opioid use increases methamphetamine self-administration, with co-use of these substances leading to a more rewarding effect when compared to methamphetamine use alone32,33. In contrast, use of psychostimulants such as methamphetamine has been shown to reduce the negative effects of opioid withdrawal34. These bidirectional effects may help in understanding the increase in opioid and methamphetamine co-use seen in the US.
As methamphetamine use among those receiving MOUD treatment continues to increase in the US, little is known about its effects on patient profiles and MOUD prognoses in rural MOUD programs. Using data from four community-based rural MOUD clinics in Oregon, this 1-year longitudinal retrospective cohort study is among the first to explore how methamphetamine UA results at treatment intake correlate with patient profiles and treatment response in real rural MOUD treatment settings.
In this study, 50% of all MOUD patients initiated treatment with a positive methamphetamine UA result during their intake assessment. Such a finding agrees with recent studies conducted in rural communities3 and highlights how frequently methamphetamine and opioid use co-occurs among individuals receiving MOUD in these regions. Somewhat in agreement with our initial hypothesis and previous studies35,36, we observed that patients initiating MOUD treatment with a positive methamphetamine UA result were more likely to be unemployed and to have a positive opioid UA result during treatment intake. However, most demographics and clinical profile variables – including homelessness, opioid use disorder severity, and psychiatric comorbidities – did not differ significantly, suggesting that the impact of methamphetamine UA status at treatment intake is not necessarily associated with a more severe clinical profile and higher social vulnerability among those initiating MOUD treatment in rural areas. Such findings differ in existing literature, which suggests that those initiating treatment who test positive for methamphetamine are more likely to have a more severe clinical profile when compared to those with a negative UA for methamphetamine21. Most relevant studies have been conducted among urban MOUD treatment programs and may not reflect the clinical profile of and social vulnerability experienced by their rural counterparts. Overall these findings highlight the importance of conducting this type of research in MOUD treatment programs in rural areas.
Regarding our primary outcome, as expected and congruent with the literature we found that a negative methamphetamine UA result at intake was associated with better methamphetamine use prognoses over time19,31-36. However, differing from our initial hypothesis and existing literature18-22, testing positive for methamphetamine at intake did not predict a worse treatment response in terms of either opioid use over time or treatment retention. In two recently published reviews, recent methamphetamine use or having a methamphetamine use disorder was associated with both opioid use outcomes and MOUD treatment retention19,21. Notably, while these reviews included studies conducted outside of the US, the majority were conducted in MOUD treatment programs in metropolitan areas. While the reasons for this lack of association are unclear, these findings call into question the assumed homogeneity of this population in terms of behavioral patterns and response to treatment across different locales. Most specifically, our findings suggest that the existing evidence from clinical trials conducted in urban areas on methamphetamine use at intake/baseline as a predictor of worse MOUD treatment outcomes may not be representative of rural populations undergoing MOUD treatment. Further research on the impact of methamphetamine use among individuals receiving MOUD in rural treatment settings is needed to better understand how to tailor rural MOUD program strategies to address the specific needs of the populations they serve.
Strength and limitations
This study has several strengths for consideration. First, data used in this study were pooled from four different community-based treatment settings located in rural Oregon, making this study likely to be generalizable to individuals seeking MOUD in other rural areas of the US, particularly those in the Pacific Northwest. Second, the substantial representation of women (44%) and the lack of a significant effect of sex on our outcome models suggests that our findings can be generalized to both sexes. Third, because participants contributing data to this study initiated treatment between January and December 2019, our intake demographic and clinical profile findings can be interpreted in terms of not only prevalence but also incidence. Fourth, this study followed MOUD patients for up to 12 months, allowing us to explore the long-term effects of intake methamphetamine UA result on methamphetamine use, opioid use, and treatment retention among MOUD patients. Fifth, our use of methamphetamine and opioid UA results to objectively determine use or abstinence attests to the methodological rigor of these outcome measures.
This study has some important limitations that need to be accounted for when interpreting our findings. First, given the nature of this study, relevant information commonly collected in substance use disorder treatment trials (such as the Addiction Severity Index assessment and Timeline Follow Back) was not collected, limiting our ability to better understand demographic and clinical profile differences between the two groups. Second, while we used UA results collected monthly to determine methamphetamine and opioid use over time, the UA detection window is 3–5 days for both substances. As such, it is probable that participants who tested negative for one or both substances in a given month may not have been abstinent from either one or both of these substances in that same month. Third, because most participants were White, it is unclear if our findings can be generalized to individuals with other racial/ethnic backgrounds seeking MOUD treatment in rural areas. Fourth, data collection for this study occurred between January 2019 and December 2019. As such, a substantial number of participants were enrolled in treatment during the COVID-19 pandemic, a period known to have drastically altered drug availability, patterns of drug use, and MOUD treatment operations. As a result, it is likely that our findings may not be representative in terms of the clinical profile and MOUD treatment response of individuals undergoing MOUD treatment in rural settings prior to and post-COVID. Lastly, and perhaps the biggest limitation of this study, is the high dropout rates. As is common in substance use disorder treatment trials, our dropout rate was high (about 60% at month 12). While we used different ways to deal with missing data to account for this phenomenon, we acknowledge that an attrition rate of 60% is substantial and limits our ability to truly understand how methamphetamine UA results at treatment intake impacted methamphetamine and opioid use over this time.
Conclusion
Opioid and methamphetamine co-use represents a major health problem for rural communities in the US. To our knowledge, this cohort study is the first to compare the clinical profile and treatment response of individuals seeking MOUD treatment in rural settings by methamphetamine UA results at intake. While somewhat unexpected, our findings identify gaps in our current understanding of the effects of methamphetamine use on MOUD treatment response, highlighting the need for more studies to be conducted with MOUD treatment-seeking rural populations.
Funding
No funding was received for this research
Conflicts of interest
The authors declare no conflicts of interest.
References
You might also be interested in:
2005 - Recruiting undergraduates to rural practice: what the students can tell us


