Introduction
Ethiopia is among the top group of world food-aid recipients1, illustrating the failure of its agricultural system to provide food for the citizens. A wide body of literature attributes this to a mounting abnormality of rainfall distribution in the country. To overcome this challenge, the Ethiopian government encouraged an expansion of rainwater harvesting (RWH), especially in the four larger regions: Tigray, Amhara, Oromia, and Southern Nations. Officials of the Ethiopian Ministry of Agriculture reported that from 2003 to 2006, approximately 952 120 on-farm RWH ponds were constructed, irrigating over 20 000 hectares.
During the period of RWH expansion a higher level of malaria incidence has occurred in the fringes of the Ethiopian highlands. According to study reports from 20032, the year RWH was officially launched, the malaria epidemic has increase six-fold from the threshold level. Over 6.1 million cases were reported with an estimated 45 000 to 114 000 deaths3. This has prompted debate about whether RWH intervention has contributed to the resurgence and expansion of malaria in some parts of the Ethiopian highlands.
The most devastating and well-documented malaria epidemic in Ethiopia was in 1958 when there were an estimated 3 million cases with 150 000 reported deaths4. Since then epidemics have occurred at intervals of 5 to 8 years5. Currently, malaria is the major cause of mortality and the leading cause of outpatient morbidity, and is prevalent in two-thirds of the country, placing at risk 50 million of the total 77 million population3.
In the 1960s, malaria was 'controlled' in most parts of Ethiopia6,7. Recently, however, malaria has not only re-emerged, but has also expanded into previously 'malaria-free' areas. The situation is the same in other east African countries. Studies variously attribute this to factors such as global warming8,9, increased population mobility10,11, drug resistance6, expansion of dams and irrigations12-15, land use/land cover changes16, and worsening socioeconomic and nutritional conditions6.
In Ethiopia, RWH dates back to 560 BCE17. However, the practice had neither been modernized nor expanded in area. However the Ethiopian government initiated use of RWH technology recently due to an increasing dry spell incidence and recurrent crop failures. Traditional ponds used in the lowland of Ethiopia are larger (≥650 m3) and shared among communities. The recently launched RWH practice is based on small (60-120 m33) ponds, constructed on and around farmlands, and owned by households. The commonest pond are bottle-, hemisphere- and trapezoid-shaped. While all bottle-shaped ponds have a perfect-fitting cap, others are mostly coverless. While bottle-shaped and hemispherical-shaped ponds are constructed of concrete materials, the trapezoid-shaped ponds are constructed from geo-membrane plastic sheets.
Studies18-20 have examined the association of water bodies with mosquito breeding. For example, a study21 of locations close to Koka dam in central Ethiopia showed the malaria incidence to be significantly higher among people living within 3 km of the reservoir. Such evidence, however, related to lakes, rivers, dams, and irrigation systems that: (i) were located at a reasonable distance from rural dwelling units; (ii) consisted of a single pooled-water body; and (iii) were created naturally (with the exception of irrigation systems).
On the contrary, the recently promoted RWH ponds are: (i) located very close to dwelling units; (ii) smaller in size (storing water only for several months at most) but numerous in number and widely dispersed throughout rural neighbourhoods; and (iii) managed by the owner of the pond. As a result, knowledge generated for malaria and the large water-body nexus cannot necessarily be extrapolated to areas dominated by RWH ponds.
It is assumed in this study that recent ecological changes in rural settlement areas have exacerbated malaria by increasing the mosquito-friendly period in the fringes of the Ethiopian highlands. This is due to the combined effect of rising temperatures and the creation of moist surfaces that remain after the summer rain season.
This article, therefore, seeks to identify issues that shape a perceived nexus between malaria and RWH practices in central Ethiopia. It is hoped that the results will suggest an approach to malaria control measures during any future expansion of RWH ponds, and identify issues for further research.
Methodology
Data
A field survey was conducted in parts central Ethiopia after the peak malaria transmission period, from late 2005 to early 2006. Both quantitative and qualitative data were collected by household survey, focus group discussions (FGD), key informant interview, and direct observation. In consultation with Zonal agricultural and health experts, five sample districts: Tehulederie, Kalu and Bati (from the Amhara region); and Dugda Bora and Bosset (from the Oromia region) were selected using purposive and stratified random sampling techniques.
From each sample district, 60 households (30 users and 30 non-users of RWH) were selected using a stratified random sampling technique, yielding a total of 300 households (150 users and 150 non-users of RWH). Non-users were included to obtain a balanced view of the RWH intervention and to enable a comparative analysis. During a pilot survey the structured questionnaire was pre-tested on 20 households that were not included in the actual household survey; this resulted in substantial improvement of the data collection tool.
The revised questionnaire enabled the capture of data that included the household's: demographic profile; land holding; land-use management; socioeconomic profile; crop-water management; pond attributes; malaria incidence, prevalence, protection and impact, in a multiple choice format. The questionnaire was responded to by the household head (20% female). In order to guarantee confidentiality, the names of respondents were not recorded on the questionnaire and they were assured that personal information would not be passed to government officials. Questionnaire enumerators were recruited from the respective districts, based on their knowledge and prior experience.
The FGD consisted of five to eight users and non-users of RWH, including youths, adults, elders and women. Three FGD were conducted in each sample district. All discussions were led by the principal and assistant researchers. During FGD, perceptions and opinions regarding food security; historical and current malaria prevalence; types and efficiencies of control measures; the role of RWH practices on the household economy and health; and an appraisal of government policies were presented for group discussion. An attempt to compute income differential between users and non-users of RWH was unsuccessful. This is because farmers are hesitant to disclose such sensitive financial information.
The study area
The study area lies in the central part of Ethiopia (Fig1). It is characterized by high rainfall variability, widespread land degradation processes, moderate to high population density, frequent episodes of food shortages, and a prevalence of malaria. Due to recurrent food shortages, the government has widely promoted RWH interventions in this area. Figure 2 shows the temporal patterns of RWH pond expansion17. Table 1 shows that the crude population density is higher in the northern than in the southern section of the study area.
Table 1: Statistics from the areas studied
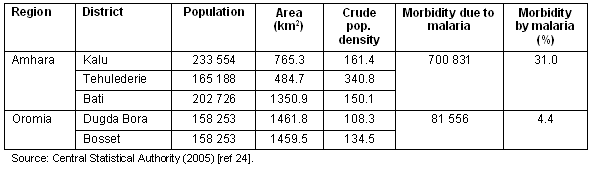
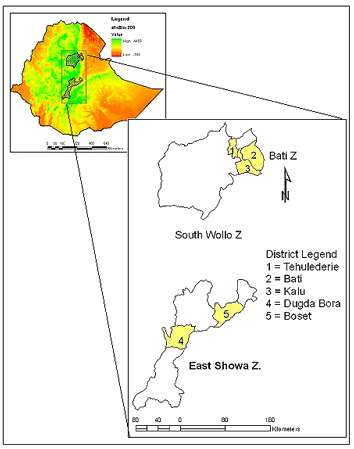
Figure 1: Location of the surveyed districts in Ethiopia.
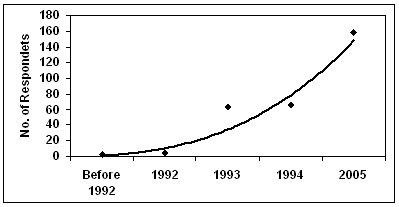
Figure 2: Patterns of rainwater harvesting pond expansion in the surveyed areas.17
The elevated parts in the northwest of the study area have rarely been exposed to malaria and their inhabitants have a low level of immunity. However, the low-lying parts in the eastern section of the study area have long been known as malaria infested. In the study area, two malaria transmission seasons take place (after the 'big' [mid-June to mid-September] and 'small' [mid-February to end of May] rain seasons).
Due to poor health coverage in Ethiopia, the majority of households in the study area do not have adequate access to early diagnosis and treatment of malaria. This is partly due to the overwhelming proportion of population residing in rural areas. Approximately 88.5% and 86.7% of the population in the Amhara and Oromia regions, respectively, lives in rural areas22. The response by communities to most malaria episodes begins with home treatment, usually with antimalarial drugs obtained from different sources, and this may result in a delay in seeking treatment from the few available health centres.
Results and Discussion
Changes in mosquito ecology
Currently, the malaria transmission period in the surveyed areas ranges from one to 4 months (Fig3). The reported transmission period is exceptionally long in Dugda Bora district. Almost all focus group participants had the conviction that the malaria transmission period has stretched in recent years.
Figure 3: Average duration (months) of malaria transmission season (reported by respondents).
In most parts of central Ethiopia, summer ('big rain') season lasts from mid-June to mid-September. Due to the combined effect of moist and warmer conditions in the months of mid-September to November, mosquitoes thrive. This peak malaria transmission period was once limited in duration by two factors. First, the daily minimum temperature is lower in the months November to January. Second, the land becomes drier. Recently, however, the influence of these two factors has weakened. Both scientific studies23 and sample respondents confirmed that daily minimum and daily maximum temperatures have been increasing. When these warmer conditions combine with RWH pond-generated moist surfaces, an extra period of malaria transmission is created (Fig4). Another study confirmed that increased vectors following irrigation can lead to increased malaria in areas of unstable malaria transmission, where communal immunity is low24.
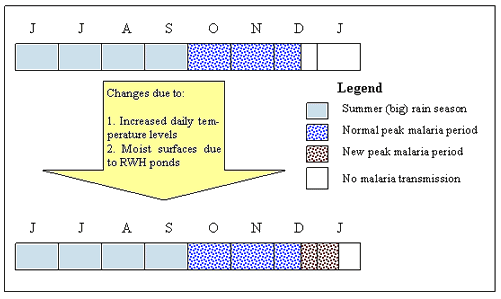
Figure 4: Changes in the malaria transmission period in the study area (source: synthesis of respondents' information). JJASONDJ, months June to January; RWH, rainwater harvesting.
With respect to the causative factors, however, the respondents' views varied significantly. While the majority of focus group participants supported this view of local malaria epidemiology, some linked it to religious causes. In this view, the rise of wrongdoing committed by this generation has saddened God, and he is 'punishing' those localities by various hazards, including the prolonged malaria transmission period.
Malaria was present before the launch of RWH in the study area; however, elders stated that it was not considered 'serious' in the past. The focus group participants in Dugda Bora district recalled an adage from approximately 30 years ago: 'As there is no fire sparkling from the cattle dung, there would not be serious sickness from malaria'. Now, malaria has become the greatest cause of death in the district. The participants generally attribute the changes in severity of malaria to: (i) the growing local 'desertification' (defined by local respondents as the depletion of natural forests coupled with an increasing air temperature and shortening of rainy seasons, that results in lower land productivity); and (ii) the weakened resistance of people to malaria. By their account, the diminishing nutritional intake of the majority of households in the district has also intensified the severity of malaria.
Figure 5 shows how the malaria-RWH nexus is perceived by sample respondents. Compared with the northern area, respondents in the southern study area strongly blamed the RWH intervention for the resurgence and expansion of malaria in their localities. Such differences could be attributed to changes observed in the local soil moisture regimen. In drier environments, such as in the south, temperature is not a mosquito-limiting factor throughout the year, rather it is the moisture conditions that control the prevalence of malaria. As is depicted (Fig3), whenever such land acquires moisture it becomes conducive to mosquitoes. Therefore, the role of RWH-induced moist surfaces on the thriving of mosquitoes and subsequent expansion of malaria incidence is more pronounced in the southern than northern study areas.
When analysis was made of users and non-users of RWH, 15% and 60% of users and non-users, respectively, attributed the rising incidence of malaria to RWH expansion in their localities. Such differences may be linked to the nutritional and economic merits obtained from RWH-irrigated farming systems. The view of the majority of focus group participants where malaria has little impact on households enjoying an adequate and balanced diet, conforms with other studies18, which stated that households that practised irrigated agriculture in some African countries have witnessed an increased malaria incidence yet have withstood its impact by the greater wealth created by these schemes.
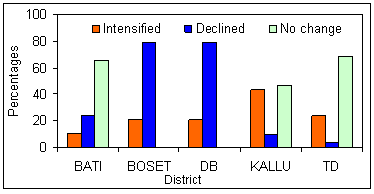
Figure 5: Opinion of respondents by district on the expansion of malaria due to rainwater harvesting pond proliferation.
Malaria and pond locations
Approximatley 80% of the RWH ponds in the surveyed area were located within 10 m of the owners' houses, showing a preference for ponds to be erected in owners' backyards, rather than in proper farmlands. According to the information obtained from focus group participants and other key informants, the distance of RHW ponds from dwellings is mostly defined by the owners.
A preference for ponds nearer houses has two possible explanations. The first relates to existing rural land tenure insecurity in Ethiopia. In the past couple of decades, the government has periodically redistributed agricultural lands with the prime intent of benefiting landless youths. Most focus group participants had bitter experience of such programs, having lost part of their land and their investment on it (eg trees, terraces). To avoid the loss of expensive ponds to land redistribution, farmers have preferred ponds near to their dwellings. Second, the location of ponds close to houses makes effective use of the household labour force, for example, women can simultaneously work on irrigated crops while cooking and taking care of children. In this way, RWH irrigated 'high value' crops can also be protected from thieves and animals by children and elders.
However, when sample households were questioned about their preferences for the likely location of future RWH ponds, a different response was obtained. As depicted (Fig6), 90% and 88% of non-users and users of RWH, respectively, preferred installing future RWH ponds at distant locations. The FGD identified two important associated issues. First, the relaxation of the government's restrictive land policy (in the 2005 a land proclamation provided better ownership security than ever before) and, related to this, the decision by Amhara and Oromia states to terminate the infamous land redistribution program. Such measures have prompted respondents to invest labour, money, and time on lands located far from dwellings. Second, both users and non-users of RWH have realized there are problems of disease and accident related to closely installed ponds. Distant ponds are understood to have a 'distance decay' effect on the concentration of mosquitoes in the vicinity of residential areas.
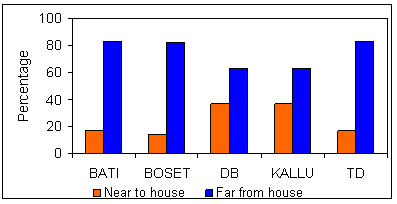
Figure 6: Preferences of farmers by district regarding the future location of their ponds.
Malaria and pond types
Data summarized from the questionnaires show that over 60% of RWH ponds are uncovered. Figure 7 shows the commonest pond cover types in the surveyed area. Initially, concern about pond coverage was related to evaporation, and the likelihood of accidents involving children and domestic animals falling into the water. It was only later that concern included the problem of mosquito proliferation. Most of the focus group participants expressed concern about open ponds creating conditions suitable for parasitic vectors and the spread of malaria in their localities.

Figure 7: Types of pond covers in the study area. Clockwise from top left: the commonest type of pond; a cover made of thatching; a bottle-shaped pond covered perfectly with cap (expensive and rare); a covered made of corrugated iron.
During field observation it was noted that even some of the 'covered' ponds allow mosquitoes to access the water. Such poorly insulated ponds provide ideal places for mosquitoes to dwell during daylight. Most key informants expressed the opinion that poorly covered ponds are riskier than open ponds. This conforms to a study conducted in India25, where defective RWH structure was found to be an important factor in dengue vector productivity.
Malaria, RWH, and household economy
The data analysis showed that 73% of RWH users had a higher household income due to the RWH intervention. District level analysis shows a higher RWH-induced income in Bati, Bosset, and Tehulederie, where 96.7%, 79.3%, and 89.6% of RWH users, respectively, acquired a higher income. The exception is in Dugda Bora district, where 56.6% of RWH users reported 'no change' in income. This could be attributed to a higher proportion of malfunctioning ponds (30%) in Dugda Bora compared with the other districts studied.
Pond-irrigated vegetables and fruit not only add to household income, but also diversify and supplement the family diets3. According to the respondents, RWH ponds are useful not only for growing vegetables, fruits, khat (Catha edulis, a stimulant crop that is highly marketable nationally and internationally) and providing for nurseries, but also for oxen fattening and water selling.
The RWH-increased income is worthy of examination. The financial expenses related to medicating family members ill with malaria may have been contributed to by income generated from RWH ponds, undermining any financial benefits. However, it could be argued that households benefiting from adequate and nutritious (mainly vegetables) food would less likely be attacked by malaria. Other studies have confirmed that a malnutrition weakened immune system can make an individual prone to malaria, malaria-related diarrhoea, and anaemia18,19.
In the absence of detailed household economic and nutritional analyses, it is difficult to determine the relative share of RWH-generated income in the household financial balance. Although this was reported as not as high as focus group participants' prior expectations, most expressed the opinion that the benefits accrued from RWH outweighed any shortcomings.
Malaria prevention
When RWH was launched in the surveyed areas, neither the government nor the local communities anticipated the likely impact of the pond-irrigated farming system on changing local soil moisture and its eventual impact on the proliferation/resurgence of malaria. Information obtained from the FGD disclosed that some households and communities began to link malaria to RWH ponds after observing an increased population of mosquitoes in the area of dwellings and experiencing a higher incidence of malaria.
The FGD conducted in Bati, Kalu and Tehulederie districts revealed that periodic stirring of ponds (using sticks or poles) significantly disrupts and spoils the colonies of mosquitoes so that eggs and larvae are prevented from hatching. Participants believed that this local innovation had greatly reduced the density of mosquitoes in their localities. Agricultural experts in those districts also acknowledged the merits of this local wisdom and are currently diffusing the information to other parts of the region.
After the adoption of the stirring technique, RWH users realized that sediment trapping ditches (Fig8) offered mosquitoes additional and ideal places for breeding. These ditches, located a few meters from the ponds, are left open, are warmer and moist. In general, it was after a greater cost (in terms of sickness with malaria, and related expenditure on medications and lost productivity) that the linkages between mosquito and RWH ponds were understood in the surveyed area.

Figure 8: Ditches dug for trapping silt (similar to those at Bati, Amhara) are also sites for mosquito breeding.
The Ethiopian Malaria Eradication Service has been spraying pesticides (mainly DDT) in various parts of the country, if intermittently and ineffectively. However, due to policy changes, both at the international and national levels, the eradication activity has weakened since the late 1960s. In the study area, most focus group participants stated that not all villages where malaria is prevalent had benefited from the relaunching of pesticide applications. Moreover, less than 25% of sample households had benefited from the insecticide treated nets.
Most focus group participants commented that the government malaria eradication measures were not only inadequate when compared with the immensity of the problem, but also limited by the obsoleteness of its information system. Key informants in the northern section of the study area stated that DDT was sprayed only in selected pockets in the past. Those few areas were labelled 'malareous' by the Malaria Eradication Office, based on a malaria distribution map prepared in the 1960s. At that time, malaria was localized to a few low lying and water-logged areas. It was argued by most focus group participants that while malaria risk has expanded to areas of higher elevation in the time since, the government still relies on the outdated malaria maps for its spraying activities. As a result, not all households in the surveyed villages are benefiting from the DDT spraying.
Conclusions
The results of this study of 300 households, focus group participants, and key informants affirms that the launching of RWH in the surveyed areas of central Ethiopia is partly responsible for the proliferation of malaria. This is evident in the elongated peak malaria transmission period and the observed higher density of mosquitoes in and around imperfect RWH ponds.
Although government should be congratulated on its food security efforts through assisted RWH, some of its policies, especially the controversial land policy, may be singled out as the underlying factors for creating mosquito-friendly environments in and around the doorsteps of residential units. Given the planned RWH expansion and the rising trend of temperature due to global and local climate change, there is the potential for an increase in malaria unless the issue is carefully investigated. Although this study was undertaken within three years of launching RWH, it could be concluded that the issues raised here will play a pivotal role in setting the agenda for future research undertakings and government interventions.
Acknowledgments
The author acknowledges that this article evolved from his brief Rainwater Harvesting Research Project report, Forum for Social Studies. The author thanks the South Wollo and East Showa zones Agricultural Bureaus; the Natural Resource experts and development agents of the sample districts; all members of the FGDs; and the sample households. Special thanks also go to Abera G Kidan who assisted the study at various stages. At finally, the manuscript reviewers are thanked for their helpful remarks on an earlier version of the manuscript.
References
1. Samuel G. Food aid and smallholder agriculture in Ethiopia: options and scenarios. In: Proceedings, Future Agricultures Consortium Workshop. 20-22 March 2006; Sussex, UK; Institute of Development Studies, 2006.
2. Negash K, Kebede A, Medhin A, Argaw D, Babaniyi O, Guintran JO et al. Malaria epidemics in the highlands of Ethiopia. East African Medical Journal 2005; 82(4): 186-92.
3. UNICEF. Malaria in Ethiopia. (Online) 2007. Available: http://www.unicef.org/ethiopia/ET_Media_Malaria_backgrounder_07.pdf (Accessed 15 December 2007).
4. Fontaine R, Najjar A, Prince S. The 1958 malaria epidemic in Ethiopia. American Journal of Tropical Medicine and Hygiene 1961; 10: 795-803.
5. Gebremariam N. Malaria. In: ZA Zein, H Kloos (Eds). The ecology of health and disease in Ethiopia. Addis Ababa: Ministry of Heath, Ethiopia, 1988; 136-150.
6. Nchinda T. Malaria: a re-emerging disease in Africa. Emerging Infectious Diseases 1998; 4: 398-403.
7. Pascual M, Ahumada J, Chaves L, Rodo X, Bouma M. Malaria resurgence in the East African highlands: temperature trends revisited. Ecology 2006; 103: 5829-5834.
8. Lindsay S, Martens W. Malaria in the African highlands: past, present and future. Bulletin of the World Health Organization 1998; 76: 33-45.
9. Zhou G, Minakawa N, Githeko A, Yan G. Association between climate variability and malaria epidemics in the east African highlands. PNAS USA 2004; 101: 2375-2380.
10. Nega A, Meskal F. Population migration and malaria transmission in Ethiopia. In: Malaria and Development in Africa. Washington, DC: American Association for the Advancement of Science, 1999.
11. Ghebreyesus T, Witten K, Getachew A, Yohannes A, Tesfay W, Minass M et al. The community-based malaria control program in Tigray, northern Ethiopia. A review of program set-up, activities, outcomes and impact. Parassitologia 2000; 42: 255-290.
12. Tedros A, Mitiku H, Karen H, Asfaw G, Ambachew M, Mekonnen Y et al. Incidence of malaria among children living near dams in northern Ethiopia. BMJ 1999; 319: 663-666.
13. Mintesinot B, Mitiku H. Water harvesting in northern Ethiopia: environmental, health and socio-economic impacts. In: PG McCornick, AB Karma, G Tadesse (Eds). Integrated water and land management research and capacity building priorities for Ethiopia. 2002; 185-191. In: Proceedings, MoWR/EARO/IWMI/ILRI, International Workshop; 2-4 December 2002, ILRI, Addis Ababa, Ethiopia; 2002.
14. Boelee E. Malaria in irrigated agriculture. Irrigation and Drainage 2003; 52: 65-69.
15. Alemayehu T, Ye-ebiyo Y, Ghebreyesus A, Witten K. Bosman A, Teklehaimanot A. Malaria, schistosomiasis, and intestinal helminths in relation to micro-dams in Tigray, northern Ethiopia. Parassitologia 1998; 40: 259-267.
16. Walsh J, Molyneux D, Birley M. Deforestation: effects on vector borne disease. Parasitology 1993; 106: 55-75.
17. Fattovich R. Remarks on the pre-Axumite period in Northern Ethiopia. Journal of Ethiopian Studies 1990; 23: 1-33.
18. Ijumba J, Lindsay S. Impact of irrigation on malaria in Africa: paddies paradox. Medical and Veterinary Entomology 2001; 15: 1-11.
19. Keiser J, de Castro M, Maltese M, Bos R, Tanner M, Singer B et al. Effect of irrigation and large dams on the burden of malaria on a global and regional scale. American Journal of Tropical Medicine and Hygiene 2005; 72: 392-406.
20. Diuk-Wasser M, Toure M, Dolo G, Bagayoko M, Sogoba N, Sissoko I et al. Effect of rice cultivation patterns on malaria vector abundance in rice-growing villages in Mali. American Journal of Tropical Medicine and Hygiene 2007; 76: 869-874.
21. Lautze J, McCartney M, Kirshen P, Olana D, Jayasinghe G, Spielman A. Effect of a large dam on malaria risk: the Koka reservoir in Ethiopia. Tropical Medicine & International Health 2007; 12(8): 982-989.
22. Central Statistical Authority. Ethiopia: Statistical Abstract, 2005. Addis Ababa: Central Statistical Authority, 2005.
23. Muna M. Variations and trends in observed temperatures in the Ethiopian highlands (1973-2003).
24. Ijumba JN, Lindsay SW. Impact of irrigation on malaria in Africa: paddies paradox. Medical Veterinary and Entomology 2001; 15(1): 1-11.
25. Mariappan T, Srinivasan R, Jambulingam P. Defective rainwater harvesting structure and dengue vector productivity compared with peridomestic habitats in a coastal town in southern India. Journal of Medical Entomology 2008; 45: 148-56.
