Introduction
Childhood obesity rates have reached epidemic proportions in the United States of America. Currently, 17% of children aged 2-19 years are overweight, as defined by having body mass index (BMI) values at or above the 95th percentiles for age and sex1. Some age groups, such as elementary school children, have seen more dramatic increases than others. For example, the percentage of children aged 6-11 years who were overweight in 1970-1974 (4%), more than tripled in 1999-2002 (15.8%)2. Although more recent National Health and Nutrition Examination Surveys estimates suggest that these rates have stabilized for the first time since 2003, overweight prevalence for 6-11 year-old children still greatly exceeds the 5% target goal established by Healthy People 20103,4. (For simplicity, in this study the general terms 'overweight' and 'obesity' will be used interchangeably. When referring to specific BMI categories, however, the Center for Disease Control and Prevention's (CDC) preferred terminology of 'at risk for overweight' and 'overweight' will be used).
Indications exist that the US obesity epidemic affects some regions more acutely than others. In rural areas, where significant health and economic disparities abound, estimates of obesity often exceed national averages5,6. State and regional investigations of high BMI prevalence have found rates ranging from 17% to 25.9% in rural areas6-8. In their re-analysis of the 2003 National Survey of Children's Health (NSCH), Lutfiyya and colleagues determined that overweight children had a 25% greater likelihood of living in rural rather than urban areas9. In another analysis of the NSCH dataset, Liu and colleagues found a significantly higher incidence of overweight in rural (16.5%) versus urban children (14.3%)10. Estimates such as these have led some to propose that rural residency itself is a risk factor for pediatric obesity9.
The consequences of this epidemic are myriad. Childhood obesity places children at risk for a host of health conditions, including hypertension, impaired glucose tolerance, type 2 diabetes mellitus, chronic inflammation, sleep apnea and orthopedic complications11,12. The psychological consequences of obesity are no less formidable and include diminished quality of life, low self-esteem, and body image dissatisfaction13,14. Epidemiological research suggests that childhood obesity endures into adulthood, making early health risks a chronic reality15-22. Given the severity and course of obesity throughout the lifespan, it is not surprising that national healthcare costs related to the disease approach US$100 billion per year19.
Obesity is a complex, multidetermined condition. Numerous etiological factors, including genetic variability, basal metabolic functioning, environmental influences, and poor health behaviors have been proposed20,21. Direct causal mechanisms for obesity are difficult to isolate, but several correlates and risk factors have emerged in the literature. Several studies have found that low socioeconomic status (SES), for example, is associated with higher risk of obesity in certain populations9,22,23. Findings regarding gender and obesity are mixed, although some evidence suggests a somewhat higher risk of overweight for boys than girls6,10. Modifiable risk factors have also been proposed, including frequent consumption of fast food and physical inactivity24,25. In addition, television viewing has emerged as an important correlate of pediatric obesity, with one study identifying a fourfold risk of becoming overweight for children who watch 5 hours or more of television per day, compared with those who watch 2 hours or less26,27.
This study presents the findings of a school-based BMI screening program targeting elementary school children living in a rural Appalachian community in southeastern Ohio. County-wide estimates of high BMI (≥85th percentile) were obtained to understand the health status and needs of this pediatric community and to compare prevalence rates with national averages. An additional aim was to identify subpopulations of children who may warrant clinical intervention due to demographic and behavioral risks factors of high BMI.
Methods
Study population
Data were collected in Athens County, USA, one of 29 rural Appalachian counties in southeastern Ohio. High rates of unemployment and poverty classify Athens as one of the poorest counties in the state28. In 2006, the county's total population was 62 062, approximately 4344 (7%) of whom were children aged 6-12 years. The county's predominant race/ethnicity is white (94.3%)28. At the time of the study approximately 4003 children were enrolled in the county's 11 elementary schools. School enrollment reports for 2006-2007 indicated that an average of 52.3% of students (range 20.4% to 71.6%) were eligible to receive meals assistance from the National School Lunch Program.
Data collection procedure
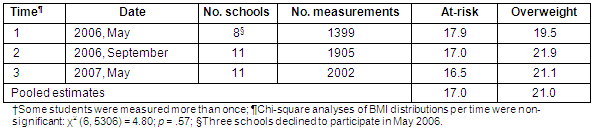
The lead investigator obtained consent from school officials to conduct repeated BMI screenings for enrolled students. With permission from each school obtained, opt-out consent forms were then sent to parents describing the study and inviting their children's participation. Children were informed of the study by school personnel and were given the opportunity to decline participation at each of the 3 data collection sessions. All 11 elementary schools consented to participate in the study but, due to scheduling conflicts, 3 schools deferred participation until the second data screening. Thus, 8 schools participated in the first collection (May 2006), while all 11 schools participated in the second (September 2006) and third (May 2007). Approximately 2000 individual children (aged 6-11 years) participated in the study, yielding a total of 5306 height and weight measurements obtained from three screening sessions (Table 1).
Table 1: Individual and pooled height and weight measurements obtained from three data collection periods (n = 5306)?
An optional Health Information Survey (HIS) was administered to parents as part of the informed consent package. The HIS is a 17-item self-report questionnaire designed by the lead author to identify demographic and behavioral risk factors associated with BMI. The initial items of the HIS elicit standard demographic information (eg income, health insurance status, household composition). Subsequent items concern nutritional choices and habits ('What types of foods do you and your family usually eat? Check all that apply' and 'How many times a week does your family buy fast food?'), recreational activities ('What type of activities do your children and family enjoy? Check all that apply') and health information ('Does anyone in your family have the following conditions? Check all that apply' and 'How many people in your household use tobacco?').
Although height and weight measurements were collected 3 times in the course of the study, the HIS was administered once, prior to the first screening; thus, it could be matched with BMI measurements obtained in the first visit only. Caregivers for a subset of this population (n = 291) completed the HIS. Return rate for this optional survey was 20.8%.
Measurements were taken by a team of medical students, interns and residents working under the supervision of the lead author. On each designated screening day, approximately six research team members obtained heights and weight measurements for all assenting students. Measurements were taken in the privacy of the teachers' lounges, which were vacated for the duration of the screening. Students were measured without coats and shoes to prevent distorted measurements.
Investigators chose to obtain 3 observations to ensure reliability of data to provide an accurate estimate of high BMI in the study area. Because the primary aim of this study was to reliably estimate high BMI prevalence rather than to track its incidence, no attempts were made to monitor changes over time in individual participants' BMI measurements.
Four digital floor scales (Conair Corp; East Windsor, New Jersey, USA; model #WW17) were used to obtain participants' weights. Weight measurements were rounded to the nearest 0.1 kg. Scales were recalibrated each day of the data collection. Heights were measured with 4 portable stadiometers (Seca, Los Angeles, CA, USA). To ensure consistency of measurements, the same 4 floor scales and stadiometers were used at each BMI screening.
Data analyses
Following conventions set by the CDC, BMI was calculated as weight in kilograms divided by height in meters23. The BMI scores were classified by the following categories: underweight (BMI ≤5th percentile); normal weight ( 5th≤ BMI <85th percentiles); at risk for overweight 85th≤ BMI ≥95th percentiles); and overweight (>95th percentile) using the 2000 CDC growth charts for gender and age6. BMI percentiles were calculated and plotted using the syntax for the statistical program SAS, as provided by the CDC30.
Chi-square tests of independence between visits and nutritional indicators revealed no significant differences in BMI measurements by time (alpha = .05). Thus, data for the 3 visits were combined for analyses. One sample tests for categorical variables (binomial test following z approximations) were used to compare prevalence of overweight and at risk of overweight in our sample with that of the entire population. Population estimates for comparison were obtained from Ogden et al. (2008)3. Logistic regression was used to determine risk factors for being classified as overweight or at risk of overweight. The software SPSS v16 (SPSS Corp, Chicago, IL, USA; http://www.spss.com/) was used for data analysis. Alpha was set at 0.05.
Results
The number of height and weight measurements obtained for each of the 3 data collection periods is presented (Table 1). Analyses of the distribution of nutritional status per time of data collection showed no statistically significant differences over the 3 time periods, χ2 (6, 5306) = 4.80, p = 0.57. Thus, data from each time period were combined to yield a more comprehensive estimate of high BMI (≥85th%) in the sample. BMI prevalence rates per data collection time are provided (Tables 1-3) for those interested in the breakdown of BMI by time. This article only discusses findings based on the pooled estimates.
Table 2: Per cent local and national prevalence of at risk for overweight and overweight according to sex, reported by data collection visits
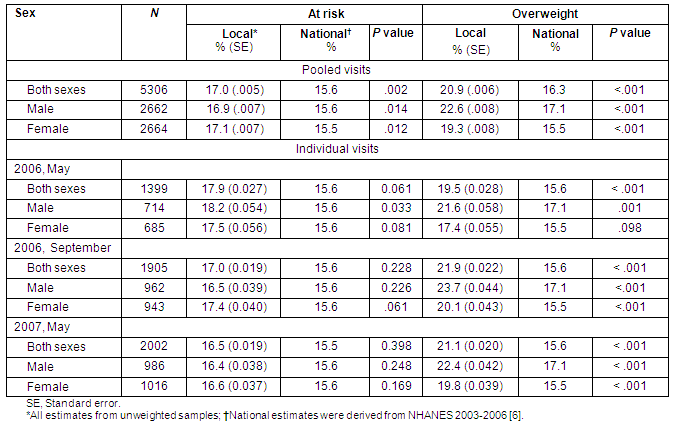
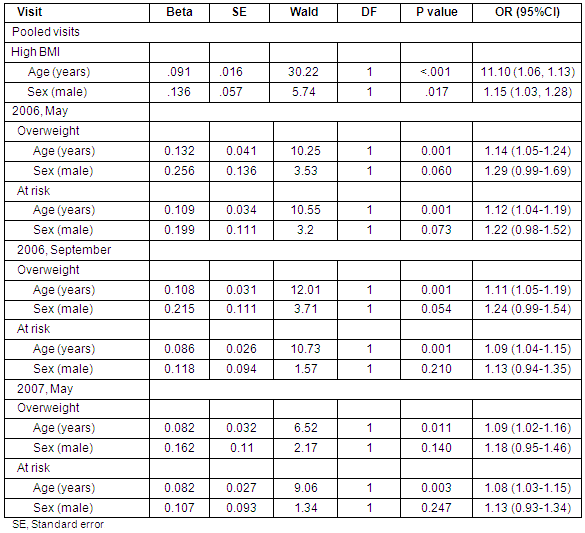
Table 3: Logistic regression of high body mass index (≥85th percentile) categories on age and sex
National versus local prevalence of high BMI categories stratified by age and gender is presented in Table 2. Collapsed across age and sex, results indicate a significantly greater prevalence of high BMI in the rural children studied versus national averages. The 17% of Athens County children classified as at risk for overweight (BMI ≥85th and <95th percentiles) exceeds national estimates (15.6%), p <0.001. Similarly, the prevalence of overweight children (BMI ≥95th percentile) in Athens County (20.9%) is significantly higher than that of the national population (15.6%), p<0.001. As shown in Table 2, these findings did not differ by gender; significantly greater prevalence rates of high BMI were found in males and females relative to national averages.
The relationship of demographic variables with high BMI (≥85th percentile) is presented (Table 3). Univariate logistical regressions indicate that both age, χ2(1) = 28.26, p <0.001, OR = 1.11 and male gender, χ2(1) = 10.65, p = 0.003, OR = 1.23 are significantly associated with the prevalence of high BMI (≥85th percentile) in the sample. In examining the 2 categories of high BMI separately, however, gender and age were found to be significantly associated with overweight (BMI ≥95th percentile). Neither variable is significant for children in the at risk for overweight category. As can be seen (Table 3), higher proportions of males (22.6%) than females (19.3%) were significantly overweight.
Demographic and behavioral factors associated with the odds of having high BMI for age are presented (Table 4). Data for these analyses were drawn from an optional HIS completed by 291 parents and caregivers of participants prior to the first data collection session. A univariate χ2 test of independence was run for the blended category of high BMI, rather than for the at risk for overweight and overweight categories separately, because the latter 2 analyses yielded insufficient cell memberships (<5%) to meaningfully interpret the results. The necessity to pool the at risk for overweight and overweight categories in order to obtain sufficient power to run analyses suggests that a higher proportion of respondents had children with normal-range BMI (>85th percentile). Thus, the HIS findings may provide additional insights into the health habits of this population but their significance should be interpreted cautiously, given the limitations outlined above.
Table 4: Demographic and behavioral factors associated with the odds of having high body mass index
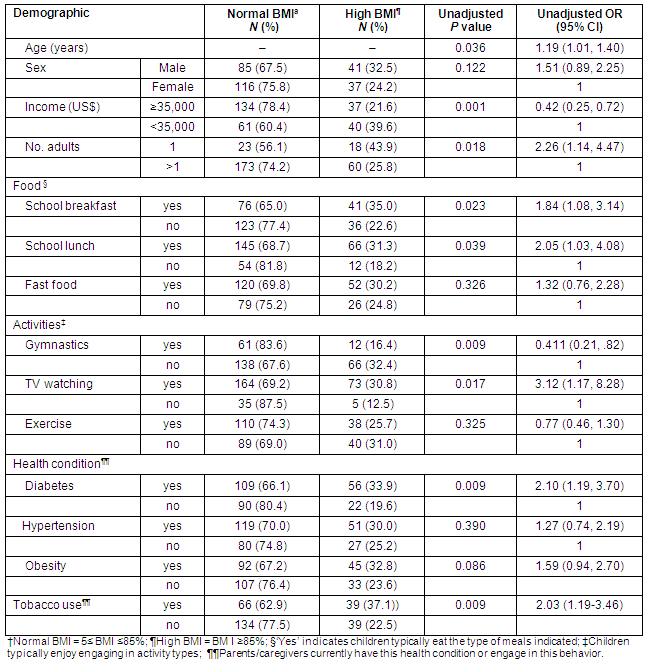
Significant correlates of high BMI for this subset of the sample included high BMI include male gender, television viewing, parental diabetes and tobacco use in the household. In addition, eating school breakfasts and lunch were also positively associated with high BMI. In contrast, several factors were associated with lower odds of having high BMI, including higher income (≥$35K), having more than one caregiver/parent in the home, and participating in gymnastics.
Discussion
A school-based BMI screening program was initiated to estimate the prevalence of high BMI in 6-11 year-old children living in a rural Appalachian region. The major findings from this study are: (i) the prevalence of childhood overweight and at risk for overweight in our rural Appalachian sample is significantly higher than national averages; (ii) sex is significantly associated with prevalence of high BMI, with a greater likelihood of boys being overweight than girls; (iii) age is significantly related to prevalence of high BMI, with older children more likely to be overweight than younger; and (iv) demographic and behavioral correlates of high BMI in this sample include lower SES, parental smoking, eating meals at school, and television viewing. Factors inversely related to high BMI include higher SES, having more than one caregiver in the home, and participation in gymnastics.
Consistent with many national, state, and regional epidemiological studies, obesity is highly prevalent in our sample of rural elementary school children6,10,31. The 20.9% of participants classified as overweight (BMI ≥95th percentile) exceeds national estimates by more than 4%. A narrower but still significant margin exists between the 17% of rural children identified as at risk for overweight and the national average (15.6%) for this age group. That these higher-than-national prevalence rates are true for boys and girls alike further emphasizes the pervasiveness of obesity in this county; all children are affected, although some subpopulations have higher prevalence rates and greater associated risks than others.
Estimates of the extent of high BMI in this sample varied by sex. Among the children classified as at risk for overweight, boys and girls alike demonstrated similar prevalence rates (16.9% and 17.1%, respectively). Gender differences did occur, however, among overweight children, with prevalence of overweight in boys (22.6%) being significantly higher than in girls (19.3%). Given these finding, it is not surprising that male gender emerged as a significant risk factor for overweight, with boys 23% more likely to develop overweight than girls. This higher prevalence of obesity in boys is consistent with previous epidemiological investigations. To the best of our knowledge, however, there are no developmental or genetic reasons for elementary-school aged boys to have such high prevalence of, and risk for, obesity7,22. More research is needed to identify possible factors that mediate the relationship between male sex and obesity, and also to determine if an interaction exists between regional residence and sex, such that high overweight prevalence in boys is part of a general trend in rural areas or is more specific to particular regions. Nevertheless, this finding furthers our objective of identifying local populations of children whose weight status warrants clinical intervention.
In addition to examining sex differences in the distribution of high BMI, the present study looked at prevalence rates by year of age. Collapsed across sexes, concentrations of high BMI by age were found to vary according to level of BMI. The percentage of children classified as at risk for overweight, for example, fluctuated by year, with age 9 years (14.7%) having the lowest prevalence. Ten and 11 year olds constituted the highest proportion of children at risk for overweight and were the only two ages in which BMI rates were significantly higher than the national average. Interestingly, the prevalence of overweight children followed a different pattern. Six year olds in our sample demonstrated the lowest prevalence of overweight (14.3%) relative to other ages; in addition, this rate was significantly less than the national average (17%). Ages 7 to 11 years, however, demonstrated a near linear increase in prevalence of overweight.
Although the design of this study is not longitudinal and precludes extrapolation, these findings suggest that a relatively large proportion of children in Athens county will enter middle school already overweight. This is alarming for two reasons. First, evidence exists that overweight children are more likely to remain overweight than their at risk for overweight normal weight counterparts32. Second, obesity that is carried into adolescence is more likely to endure into adulthood; at least one study, for example, found that that 33% of overweight boys between the ages of 8 and 13 years become obese adults32.
The design of this study allows us to speculate about, but not establish possible reasons for the findings. The HIS highlighted several health, lifestyle and behavioral factors that may explain the BMI prevalence rates. As with numerous other studies, a positive association was found among high BMI and habitual television viewing, sedentary behavior, and parental diabetes, and tendency to eat school breakfasts and lunches16-23. These correlations make intuitive sense and corroborate the findings of previous research, but must be interpreted with caution because they represent only a subset of participants and may not readily generalize.
Of note, in examining the response rates of the optional HIS, it was discovered that a disproportionate number of respondents had children in the normal BMI range (<85th percentile). Although the reasons for this unbalanced representation of respondents can only be speculative, it could be that parents of overweight children are less inclined to disclose nutritional and health habit information due to shame, guilt, or fears of being 'blamed' for their children's obesity. Whatever the reason may be, this finding illuminates a potential consideration for other pediatric obesity researchers and interventionists: namely, that the participants you seek to reach may be the hardest to access.
The findings of this study must be interpreted within other limitations. In conducting multiple school-based BMI screenings, it was necessary to balance methodological rigor with feasibility. Accordingly, there was no attempt to track the changes in individual participants' BMI over the 12 months of the study. Thus, some participants were measured more than once across the 3 screening sessions, but the distribution of BMIs per measurement were homogenous enough to allow all the data to be pooled.
In addition, although BMI is the most pragmatic way to gauge pediatric obesity, it not a direct measure of adiposity16. Although our findings would have been strengthened by including waist circumference measurements, for example, we deemed this unfeasible given the time constraints imposed by the schools and the sheer number of students targeted.
Despite these limitations, this research contributes to current knowledge of obesity in children. Our data concur with prevailing conclusions in the pediatric obesity literature, especially regarding higher-than-national prevalence of overweight in rural areas. In addition, this study adds a unique perspective, for within the vast body of obesity research there is a paucity of studies devoted specifically to the health of Appalachian children. In a related vein, this study adds to the relatively meager body of research on pediatric obesity in remote, underserved areas. Finally, we cite our methodology as major contribution of this study. School-based BMI screenings are the most efficient way to gather cross-sectional and longitudinal data about the prevalence and incidence of pediatric obesity. It is the hope (and recommendation) of the authors that other rural researchers and clinicians are not daunted by the complexities of conducting large-scale screenings within schools. Indeed, the public health benefits of having this information - for children, parents, and health service providers - outweighs the logistical considerations of conducting community-based research.
Conclusion
Given the pervasive poverty, health disparities and premature mortality found in Appalachia, it is critical to identify and address the unique health needs of its youngest residents33,34. Appalachian communities tend to be remote and isolated, both geographically and culturally, from the rest of the nation. Little is known about how health risks and conditions such as obesity develop throughout Appalachian residents' lifespan6,35. Moreover, although most Appalachian regions are rural, not all rural areas are Appalachian; thus, we cannot readily assume that pediatric obesity in Appalachian areas approximates obesity in rural America. The findings of this study indicate that the prevalence and possible correlates of childhood obesity in this Appalachian region are, in fact, comparable with other rural, non-Appalachian areas. Whether or not other regions follow suit is the task of future epidemiological research. By focusing on one piece of this larger health picture, however, this study provides additional insights into this population, and helps to locate Appalachia within the larger context of a national obesity crisis.
Acknowledgements
This work was supported by the Ohio University College of Osteopathic Medicine's Research and Scholarly Affairs Committee and by the Centers for Osteopathic Research and Education (CORE). The authors are particularly grateful to the staff, students and parents of the Athens City and County elementary schools who participated in the study.
References
1. Stoddard SA, Kubik MY, Skay C. Is school-based height and weight screening of elementary students private and reliable? The Journal of School Nursing 2008; 24(1): 43-48.
2. National Center for Health Statistics. Health, US, 2008. Hyattsville, MD: US Public Health Service, 2008. Available: http://www.cdc.gov/nchs/data/hus/hus08.pdf (Accessed 14 October 2009).
3. Ogden CL, Carroll MD, Flegal KM. High Body mass index for age among US children and adolescents, 2003-2006. Journal of the American Medical Association 2008; 299(20): 2401-2405.
4. US Department of Health and Human Services. With understanding and improving health and objectives for improving health. Healthy people 2010, 2nd edn. Washington, DC: US Government Printing Office, 2000.
5. Merten MJ, Wickrama KAS, Williams AL. Adolescent obesity and young adult psychosocial outcomes: gender and racial differences. Journal of Youth and Adolescence 2008; 37(9): 1111-1122.
6. Demerath E, Muratova V, Spangler E, Li J, Minor VE, Neal WA. School-based obesity screening in rural Appalachia. Preventive Medicine 2003; 37: 553-560.
7. Tudor-Locke C, Kronenfeld JJ, Kim SS, Benin M, Kuby M. A Geographical comparison of prevalence of overweight school-aged children: the National Survey of Children's Health 2003. Pediatrics 2007; 120(4): 1043-1050.
8. Davis C, Flickinger B, Moore D, Bassali R, Domel-Baxter S, Yin Z. Prevalence of cardiovascular risk factors in school-children in a rural Georgia community. American Journal of the Medical Sciences 2005; 330(2): 53-59.
9. Lutfiyya MN, Lipsk MS, Wisdom-Behounek J, Inpanbutr-Martinkus M. Is rural residency a risk factor for overweight and obesity for US children? Obesity 2007; 15(9): 2348-2356.
10. Liu J. Urban-rural differences in overweight status and physical inactivity among US children aged 10-17 years. Journal of Rural Health 2008; 24(4): 407-415.
11. Hughes AR, Reilly JJ. Disease management programs targeting obesity in children: setting the scene for wellness in the future. Disease Management and Health Outcomes 2008; 16(4): 255-266.
12. Thompson DR, Obarzanek E, Franko DL, Barton BA, Morrison J, Biro FM et al. Childhood overweight and cardiovascular disease risk factors: The National Heart, Lung, and Blood Institute Growth and Health Study. Journal of Pediatrics 2007; 150(1): 18-25.
13. Braet C, Mervielde I, Vandereycken W. Psychological aspects of childhood obesity: a controlled study in a clinical and nonclinical sample. Journal of Pediatric Psychology 1997; 22(1): 59-71.
14. Davison K, Birch L. Weight status, parent reaction, and self-concept in five-year-old girls. Pediatrics 2001; 107(1): 46-53.
15. Thompson DR, Obarzanek E, Franko DL, Barton BA, Morrison, J, Biro FM et al. Childhood overweight and cardiovascular disease risk factors: The National Health, Lung and Blood Institute Growth and Health Study. Journal of Pediatrics 2007; 150(1): 18-25.
16. Freedman DS, Dietz WH, Srinivasn SR, Berenson GS. Risk factors and adult body mass index among overweight children: The Bogalusa Heart Study. Pediatrics 2009; 123(3): 750-757.
17. Steinberger J, Moran A, Hong C, Jacobs D, Sinaiko A. Adiposity in childhood predicts obesity and insulin resistance in young adulthood. Journal of Pediatrics 2001; 138: 469-473.
18. Lagstrom H, Hakanen M, Niinikoski H, Viikari J, Ronnemaa T, Saarinen M et al. Growth patterns and obesity development in overweight or normal-weight 13-year old adolescents: The STRIP study. Pediatrics 2008; 122(4): 876-883.
19. Rao G. Child obesity. Amherst, NY: Prometheus, 2006.
20. Schmitz KH, Jeffry RW. Prevention of Obesity. In: TA Wadden, AJ Stunkard AJ (Eds). Handbook of Obesity Treatment. New York: Guilford Press, 2002; 515-531.
21. Wieting JM. Cause and effect in childhood obesity: solutions for a national epidemic. Journal of the American Osteopathic Association 2007; 108(10): 545-552.
22. Veugelers PJ. Prevalence of and risk facgtors for childhood overweight and obesity. Canadian Medical Association Journal 2005; 173(6) . Available: http://www.cmaj.ca/cgi/content/full/173/6/607 (Accessed 13 October 2009).
23. Booth M, Macaskill P, Lazarus R, Baur L. Sociodemographic distribution of measures of body fatness among children and adolescents in New South Wales, Australia. International Journal of Obesity 1999; 23: 456-562.
24. Watts K, Beye P, Siafarikas A, O'Driscoll G, Jones T, Davis E et al. Effects of exercise training on vasular function in obese children. Journal of Pediatrics 2004; 44: 620-625.
25. Johnson L, Mander A, Jones L, Emmett P, Jebb S. Energy-dense, low-fiber, high-fat dietary pattern is associated with increased fatness in childhood. American Journal of Clinical Nutrition 2008; 87: 846-854.
26. Robinson TN. Reducing children's television viewing to prevent obesity. JAMA 1999; 282: 1561-1567.
27. Gortmaker SL, Must A, Peterson AMS, Coldritz GA, Dietz WH. Television viewing as a cause of increasing obesity among children in the United States, 1986-1990. Archives of Pediatrics and Adolescent Medicine1996; 150: 356-362.
28. US Census Bureau. State and county quickfacts. (Online) 2006. Available: http://quickfacts.census.gov/qfd/states/39/39009.html (Accessed 6 April 2009).
29. Kuczmarski R, Ogden C, Guo S, Grummer-Strawn L, Flegal K, Mei Z, et al. 2000 CDC growth charts for the United States: methods and development. Vital Health Statistics 2002; 11(246): 1-190.
30. Center for Disease Control and Prevention. A SAS Program for the CDC Growth Charts. (Online) 2009. Available: http://www.cdc.gov/nccdphp/dnpa/growthcharts/resources/sas.htm (Accessed 15 August 2009).
31. Smith DT, Vendela MJ, Bartee T, Carr LJ. Body mass index in rural first grade schoolchildren: progressive increase in boys. Journal of Rural Health 2008; 24(1): 40-48.
32. Berkowitz RI, Stunkard AJ. Development of childhood obesity. In: TA Wadden, AJ Stunkard AJ (eds). Handbook of obesity treatment. New York: Guilford Press, 2002; 515-531.
33. Haverson J, Ma L, Harner E. An analysis of disparities in health status and ccess to care in the Appalachian region. (Online) 2004. Available: http://www.arc.gov/index.do?nodeId=2335 (Accessed 13 October 2009).
34. Behringer B, Friedell G. Appalachia: where place matters in health. Prevention and Chronic Disease 3(4). (Online) 2006. Available: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1779277/ (accessed 5 May 2009).
35. Crooks DL. Child growth and nutritional status in a high-poverty community in eastern Kentucky. American Journal of Physical Anthropology 1999; 109: 129-142.
Abstract
Introduction: In rural regions of the United States of America, estimates of pediatric obesity often exceed national averages. This problem may be particularly pronounced in Appalachian regions, where significant health and economic disparities abound. This study presents the findings of a body mass index (BMI) screening program for 6-11 year old children living in a rural Appalachian community. County-wide estimates of high BMI (≥85th percentile) were obtained to understand the health status and needs of our pediatric community and to compare obesity prevalence rates with national averages. An additional aim was to identify subpopulations of children who may warrant clinical intervention due to demographic and behavioral risks factors of high BMI.
Methods: A school-based BMI screening was conducted of 6-11 year old children in southeastern Ohio. Investigators collected 3 sets of height and weight measurements from approximately 2000 elementary school students between 2006 and 2007. Caregivers for a subset of this population also completed a health behaviors questionnaire.
Results: Thirty-eight percent of children had high BMI, with 17% at risk for overweight and 20.9% overweight. Boys were 23% more likely than girls to be overweight (χ2(1) = 95% CI = 1.08, 1.40) and 11% more likely to become overweight with each year of age (OR = 1.11, 95% CI = 1.07, 1.15). Overweight children were more likely to view television, eat meals at school, and live with a caregiver who smokes.
Conclusions: Consistent with expectations, prevalence of high BMI in this sample of rural Appalachian children exceeds national averages. Prevalence of overweight varied by age and sex; boys are particularly vulnerable to developing obesity, especially as they age. Preliminary survey data suggest that eating breakfast at home and at school and increased hours of television viewing may be associated with higher BMI, especially in younger boys.
Key words: Appalachia USA, body mass index, epidemiology, pediatric obesity, school-based screening.




