Introduction
Cervical and breast cancer are the most common malignancies among women worldwide and their incidence is rising steadily with an annual estimated 468 000 and 999 000 new cases, and 233 000 and 375 000 deaths, respectively1,2. Tragically, half of these occur in the women of developing countries where cervical and breast cancers are the leading tumours in incidence and mortality, a mortality rates are usually higher3-6.
Regular breast and cervical cancer screening interventions facilitate early detection and can dramatically reduce mortality rates from these cancers. However, in developing countries effective screening and treatment programs are unavailable to the majority of the population. Without early detection patients present in an advanced stage, reducing the opportunity for efficient treatment7,8.
Success in prevention involves effective public health programs and procedures, such as screening. To reduce mortality and morbidity, cancer screening requires persistent professional efforts that are efficient, effective, target the population at risk, and ensure that those identified can receive the necessary care. Data must be interpreted precisely to guide the process9,10.
The women at highest risk have characteristics that complicate screening. Those at high risk for cervical and breast cancer morbidity and mortality have a low education level, low income, and low health literacy; they also have the sociodemographic characteristics that most complicate screening and care. Women aged 50 years and over are more likely to be out of reach to conventional office-based cancer screening programs11,12.
The interface between those screening patients and the patients most needing screening is also complex. Cultural beliefs and misunderstandings of health behaviours are just two problem areas. In addition, a great number of women at risk of breast and cervical cancer are uninsured or under-insured.
Breast and cervical cancer are complex diseases involving a variety of genetic and environmental risk factors that can complicate a realistic and specific primary prevention strategy for the general population. Screening procedures vary for the two diseases. Mammography screening is a specific, essential step in identifying and treating of breast cancer. However the effective control of cervical cancer depends on the early detection of precancerous lesions using the Papanicolaou (Pap) test, an apparently simple test but one that depends on a range of socioeconomic variables (schooling, number of sexual contacts, parity etc) and technical complexities, including adequate preparation and reading of smear slides13,14.
Successful screening programs depend not only on participants' perceptions of the healthcare community, but also the healthcare resources available. Women from remote, rural areas face barriers that include insufficient medical services. Cancer mortality in rural areas is higher and referral occurs later than in urban areas, indicating different patterns of care for rural populations15. It was recently shown that patients travelling more than one hour had lower admission rates to a specialist cancer centre. With travel of more than 3 hours they usually found the cancer hospital facility nearest their home address but were admitted for significantly fewer days than all other groups15. This is an obvious concern for population and public health authorities.
In this context, mobile healthcare units can offer a superior screening strategy. Women from remote areas can use them to access medical care and screening tests, and for clinical examinations. Outreach health personnel in mobile units can increase awareness of cancer prevention and early detection, and also offer health education while supporting the local healthcare system16. For more than a decade, cervical and breast cancer early detection has significantly benefited from the use of mobile units in remote zones17. However, strategies to improve the uptake of both screening tests have had a poor result among lower-income, lower-educational background and older non-compliant women18,19.
Therefore, the aim of this study was to indentify strategies to improve compliance with mass screening for breast and cervical cancer in a remote, rural female population aged 20 years and over using the Mobile Unit of the Barretos County Cancer Screening Project (BCCSP). This appears to be the first report on the use of mobile units to improve mammogram uptake in South America.
Methods
The BCCSP was a designed breast and cervical cancer screening project aiming to reach women in the Barretos County region, São Paulo state, Brazil. In this region, which consists of 19 neighbouring cities mainly located in large rural areas controlled by the 9th Regional Health Administration Office (9th RHAO), there were approximately 124 000 women in the target age group. This study reports the results of the project's first year (2003-2004).
Ethics approval
Written informed consent was obtained from all participants and the study was approved by the Committee of Ethics on Research of the Cancer Hospital. Descriptive analyses were used where applicable.
Awareness of the prevention cancer strategies
The chief nurses of local facility units for each district health system had a key role in the program, being responsible for publicising the screening program. The strategies they used throughout the target areas included broadcasts by radio and loudspeaker cars, distributing flyers, pamphlets and advertising posters to local public health facilities, GP notifications and home visits by community healthcare agents (CHCAs; especially trained at a nationwide family health program of the Brazilian Ministry of Health Public Health Service). Members of the organising team met regularly with these nurses to assess screening progress and adapt strategies for use in specific cities prior to the mobile screening visits.
Before individual screening, women were interviewed and filled out a questionnaire to assess their:
- level of knowledge of breast and cervical cancer prevention
- prior experience of screening
- educational and socioeconomic background.
Educational background was classified as: none (illiterate); elementary (basic and middle); high school; or graduate level. Socioeconomic status was classified as: very poor; poor; middle; upper-middle; or upper class, according to the Brazilian Society of Marketing Research classification. This classification uses features such as housing, furniture, appliances, hygienic and sanitary conditions, shopping activities and leisure, which are regarded as easier to evaluate by questionnaire than expenses and income alone20.
The mobile unit
The mobile unit had two rooms for gynaecological examinations, a room for mammogram fitted with GE Senograph? 700T equipment, and a darkroom for film development. A satellite wireless information system database updated Cancer Hospital data in real time. This avoided screening duplication in either the mobile unit or the hospital out-patient clinic before a two-year recall interval.
On chief-nurse recommendations the mobile unit spent 2 to 5 days in each city (according to the population to be screened), and returned every 3 months. On detection of a suspicious cervical lesion, palpable breast abnormality, abnormal mammogram or Pap test, the woman was referred to the Cancer Hospital for further investigation.
Results
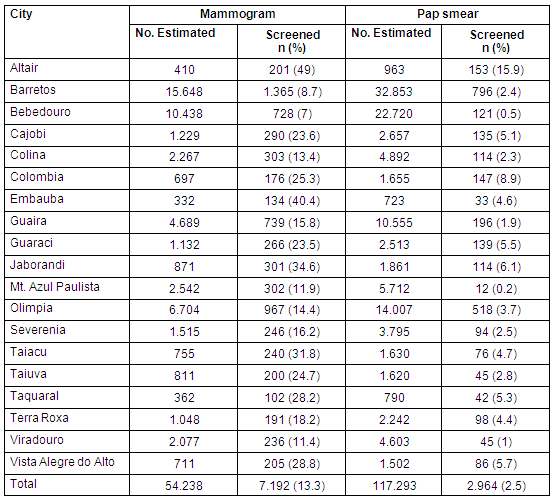
A total of 10 156 examinations were performed, comprising 7192 mammograms (71%) and 2964 Pap tests (29%). The age, educational and socioeconomic background of the screened women are provided (Table 1). A total of 3065 (43.7%) and 200 (6.7%) women had never undergone breast and cervical screening, respectively; and 1395 (19%) and 306 (10.3%) women had not been screened for more than 3 years.
Home visits by CHCAs accounted for 45.6% of all attendances, while the remainder were mostly due to radio (11.9%) and neighbourhood notifications (9.3%). Table 2 shows the overall attendance according to information strategy. Few of the cities had established Public Health Service Family Health Programme personnel or CHCA assistance. Some strategies failed to reach participants in some cities. Attendances for BCCSP breast and cervical screening according to the information strategy are detailed (Tables 3 and 4, respectively).
Of all screened women, 6643 (92%) and 2905 (99.8%) had no signs or symptoms of either breast or cervical disease, respectively. Complementary breast examinations were carried out in 431 participants (6%) and 105 (1.4%) underwent biopsy, resulting in 22 diagnosed breast cancer cases. Among the 2964 women who underwent Pap testing, 15 (0.5%) were found to have the following cytological changes: three (0.10%) atypical squamous cells of undetermined significance (ASCUS); four (0.13%) cervical intraepithelial (CIN) 1; three (0.10%) CIN 2; three (0.10%) CIN 3; and two cases of invasive squamous cell carcinoma (0.07%) diagnosed as IA2 and IB1 clinical stages). Both breast and cervical cancers were classified as clinically early-stage tumours in 45% and 100% of cases, respectively. The percentage of women effectively screened for the estimated population was higher for the mammogram group (13%) than the Pap-test group (2.5%) (Table 5).
Table 1: Age, educational and socioeconomic background distribution of screened women in the Barretos County Screening Project
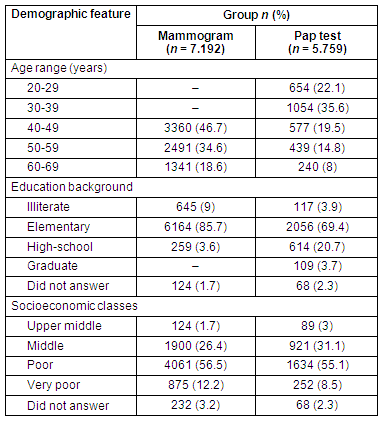
Table 2: Source of acquired information about screening in the Barretos County Screening Project, according to screening test
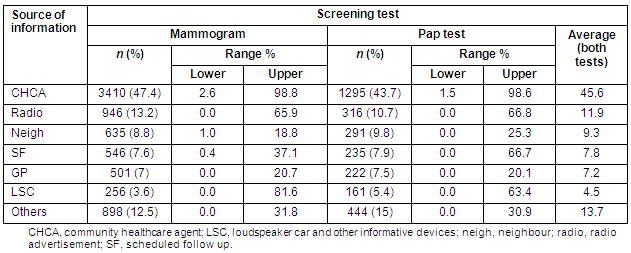
Table 3: Source of acquired information on breast cancer screening (mammogram) by city in the Barretos County Screening Project
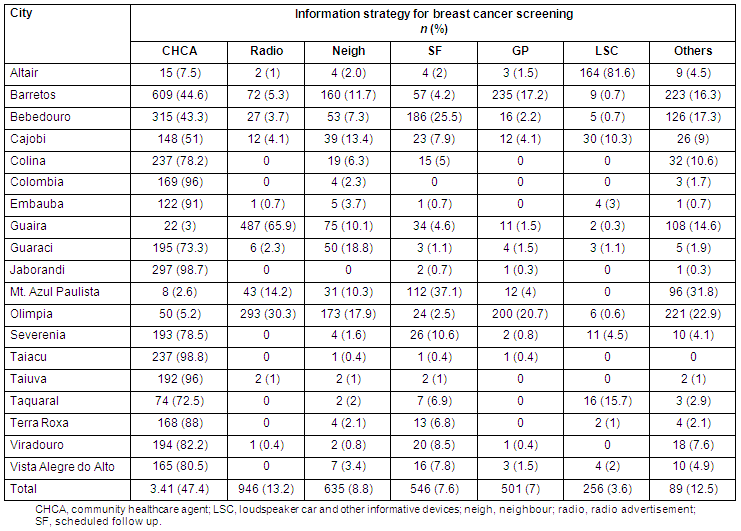
Table 4: Source of acquired information on cervical cancer screening (Pap test) by city in the Barretos County Screening Project
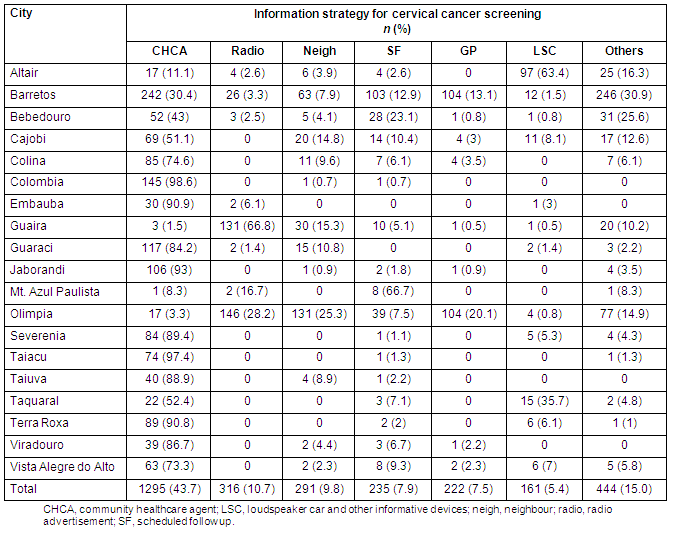
Table 5: Effectively screened population according to city and screening test
Discussion
In this study, 45% and 100% of breast and cervical cancer cases, respectively, were identified at early stages, and this is similar to other screening programs21 (pers. comm., Incentivo do Programa Agente Comunitário de Saúde da Família, 9 January 2009).
The home visits by CHCAs were most effective in improving the number of women screened, and this was most successful when performed by a local agent who was well known in the community. Local agents have the advantages of shared culture, class and language which promotes understanding, even among low educational background women22. This result is similar to the findings of other studies23,24.
The intervention strategy of using popular radio broadcasts was also found useful in our study, reaching a great number of women who encouraged their neighbours to attend. This was the main method for attendance for screening in three of the 19 cities visited. This finding is different from that of a South African study where the use of radio had little impact on screening rates25.
A combination or modification of successful strategies may be even more beneficial. For instance, the combination of a mobile unit visit that is publicised by community or lay health representatives and radio information may be quite powerful. In small cities or areas with a low population density, motorcycles equipped with a loudspeaker system may be better than radio or car broadcasts.
The coordination of screening with follow-up clinical and surgical activities was a strong point of the intervention strategy. This clustering of services presented fewer barriers to treatment than single modalities would have.
Response to screening can also involve local access issues. Mammograms are more difficult to obtain than Pap tests, and this may explain the greater number of breast rather than cervical screenings in this study26-28. Local access can encourage or discourage screening. Those screened in this study did not attribute screening to the efforts of their GPs in basic health units, who may be too busy provide information about screening. In Brazil, GP activities are related to a complex governmental program of family health, which involves prevention, diagnosis and treatment, with a focus on infectious diseases and nutrition. While cervical and breast cancer screening are within the scope of GP activities, one physician (with a nurse and 12 community agents) is responsible for the care of up to 3550 people, which can impose serious limitations on the medical care available.
The county determines which programs to implement, with many variables impacting on these decisions. The rationale for the existence of a program is to avoid overloading hospitals, but access barriers to programs still exist (eg http://www.saudeprev.com.br/psf/saopaulo/GM-648.htm). For this reason, mobile unit activities are encouraged. However, without a basic health access plan that integrates the various programs and providers, screening efforts may be complex.
Studies of screening effectiveness are important to the policy decision of whether to invest scarce funding in tertiary treatment or prevention and screening. When funding is less available, the decision may be more difficult. According to World Bank figures, health expenditure per capita in the year 2000 was US$262 in Latin America and the Caribbean compared with US$4499 in USA and US$1924 in the European Union29.
Conclusion
Ultimately solutions to health access, public health, and health screening issues involve coordination at all levels - patient, community, practitioner, system, and referral hospital. There are many obstacles to overcome and, for women, some of the greatest involve disregarding their own health status and need for care20,30,31. To avoid unacceptable health risk, well-conducted programs must bring education, testing, and the best advice possible to those in need of care. This can be accomplished by the use of mobile units coordinated with public health and an oncologic hospital.
In low-income, low-educational background female populations, a multimodal approach to community outreach strategies, especially using CHCAs and radio advertisements, can improve the uptake of mass screening.
References
1. Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. Journal of Clinical Oncology 2006; 24: 2137-2150.
2. Parkin DM, Bray FI, Devesa SS. Cancer burden in the year 2000. The global picture. Eur Journal of Cancer 2001; 37(Suppl 8): S4-S66.
3. Bulliard JL, Ducros C, Jemelin C, Arzel B, Fioretta G, Levi F. Effectiveness of organised versus opportunistic mammography screening. Annals of Oncology 2009; 20: 1199-1202.
4. Hébert JR, Daguise VG, Hurley DM, Wilkerson RC, Mosley CM, Adams SA et al. Mapping cancer mortality-to-incidence ratios to illustrate racial and sex disparities in a high-risk population. Cancer 2009; 115: 2539-2552.
5. Capocaccia R, Martina L, Inghelmann R, Crocetti E, De Lisi V, Falcini F et. al. A method to estimate mortality trends when death certificates are imprecisely coded: an application to cervical cancer in Italy. International Journal of Cancer 2009; 124: 1200-1205.
6. São Paulo Oncocentre Foundation (Fundação Oncocentro de São Paulo - FOSP): [Hospital-based Cancer Registry]. (Online) 0000. Available: http://www.fosp.saude.sp.gov.br/html/fr_dados.html (Accessed 20 August 2007; in Portuguese).
7. Fernández ME, Gonzales A, Tortolero-Luna G, Williams J, Saavedra-Embesi M, Chan W et al. Effectiveness of Cultivando La Salud: a breast and cervical cancer screening promotion program for low-income Hispanic women. American Journal of Public Health 2009; 99: 936-943.
8. Martins LF, Valente JG, Thuler LC. Factors related to inadequate cervical cancer screening in two Brazilian state capitals. Revista de Saúde Pública 2009; 43: 318-325.
9. Arbyn M, Simoens C, Van Oyen H, Foidart JM, Goffin F, Simon P et. al. Analysis of 13 million individual patient records pertaining to Pap smears, colposcopies, biopsies and surgery on the uterine cervix (Belgium, 1996-2000). Preventativ Medicine 2009; 48: 438-443.
10. Frieden TR, Myers JE, Krauskopf MS, Farley TA. A public health approach to winning the war against cancer. Oncologist 2008; 13: 1306-1313.
11. Puigpinós R, Borrell C, Antunes JL, Azlor E, Pasarín MI, Serral G et. al. Trends in socioeconomic inequalities in cancer mortality in Barcelona: 1992-2003. BMC Public Health 2009; 9: 35.
12. Menvielle G, Kunst AE, Stirbu I, Strand BH, Borrell C, Regidor E et. al. Educational differences in cancer mortality among women and men: a gender pattern that differs across Europe. British Journal of Cancer 2008; 98: 1012-1019.
13. Lawson HW, Henson R, Bobo JK, Kaeser MK. Implementing recommendations for the early detection of breast and cervical cancer among low-income women. MMWR Recommendation Report 2000; 49(RR-2): 37-55.
14. Adams EK, Breen N, Joski PJ. Impact of the National Breast and Cervical Cancer Early Detection Program on mammography and Pap test utilization among white, Hispanic, and African American women: 1996-2000. Cancer 2007; 109(2 Suppl): 348-358.
15. Baird G, Flynn R, Baxter G, Donnelly M, Lawrence J. Travel time and cancer care: an example of the inverse care law? Rural Remote Health 8: 1003. (Online) 2008. Available: www.rrh.org.au (Accessed 19 August 2009).
16. Mayo RM, Sherrill WW, Crew L, Watt P, Mayo WW. Connecting rural African American and Hispanic women to cancer education and screening: the Avon Health Connector Project. Journal of Cancer Education 2004; 19: 123-126.
17. Alexy BB, Elnitsky C. Rural mobile health unit: outcomes. Public Health Nursing 1998; 15: 3-11.
18. Gomes UA, Mauad EC, Melani AGF, Hidalgo GS, Véo CAR. Cervical cancer screening among rural Brazilian women. Cancer Detection and Prevention 2004; 313: 147.
19. P Mauad EC, Gomes UA, Nogueira JL, Melani AGF. Prevention of cervical cancer in a poor population in Brazil. Family Practice 2002; 19: 189-192.
20. Martins GA, Lintz A. Técnicas para Coleta de Dados e Informações. São Paulo: Atlas, 2000; 52-54.
21. Njor SH, Olsen AH, Bellstrøm T, Dyreborg U, Bak M, Axelsson C et al. Mammography screening in the county of Fyn. November 1993-December 1999. APMIS Suppl 2003; 110: 1-33.
22. Holanda F Jr, Castelo A, Veras TM, de Almeida FM, Lins MZ, Dores GB. Primary screening for cervical cancer through self sampling. International Journal of Gynaecology and Obstetrics 2006; 95: 179-184.
23. Suarez L, Nichols DC, Brady CA. Use of peer role models to increase Pap smear and mammogram screening in Mexican-American and black women. American Journal of Preventative Medicine 1993: 9: 290-296.
24. Morris DL, Felkner M, McLean CH. Pap screening in the Rio Grand Valley: a case study. Family and Community Health 1994; 17: 1-14.
25. Rise L, Bindman JP, Campbell OM, Imrie J, Everett K, Bradley J et al. Media interventions to increase cervical screening uptake in South Africa: an evaluation study of effectiveness. Health Education and Research 2004; 19: 457-468.
26. Wells KJ, Roetzheim RG. Health disparities in receipt of screening mammography in Latinas: a critical review of recent literature. Cancer Control 2007; 14: 369-379.
27. Schueler KM, Chu PW, Smith-Bindman R. Factors associated with mammography utilization: a systematic quantitative review of the literature. Journal of Women's Health (Larchmt) 2008; 17: 1477-1498.
28. Coughlin SS, Leadbetter S, Richards T, Sabatino SA. Contextual analysis of breast and cervical cancer screening and factors associated with health care access among United States women, 2002. Social Sciences and Medicine 2008; 66: 260-275.
29. Indicadores do Desenvolvimento Mundial 2003 [The World Bank Annual Report 2003]. Metas Globais de Combate à Pobreza estão alcance, mas somente com ação decisiva nos setores do comércio, ajuda externa e investimento nas pessoas. (Online) 2003. Available: http://www.bancomundial.org/infoannual/2003/ (Accessed: 18 November 2005).
30. Mauad EC, Gomes UA, Melani AGF, Morini SR, Denadai MV. Cervical cancer screening among urban Brazilian women. Cancer Detect Prev.2004; 00: p.147(Abst.312).
31. Agurto I, Bishop A, Sanchez G, Betancourt Z, Robles S. Perceived barriers and benefits to cervical cancer screening in Latin America. Preventative Medicine 2004; 39: 91-98.
