Introduction
Although adolescents comprise 20% of the world's population1, data on their dietary intake are limited, with the majority of research focused on children. Adolescence is a critical time for physical and social development and this accelerated growth inevitably increases energy and micronutrient needs2. Internationally, insufficient attention has been paid to improving nutrition during and after puberty3, although it has been acknowledged that nutrition and weight status during adolescence affects adult health. Approximately half the overweight (OW) adolescents and more than one-third of OW children remain obese (OB) as adults4. In Northern Greece, 31% of the adolescent boys and 21% of adolescent girls are OW/OB and this has been attributed to incorrect nutrition and the adoption of a westernized diet5. A recent nationwide study in Greece identified a lower prevalence of OW/OB, classifying 29.4% of adolescent boys and 16.7% of adolescent girls as OW/OB, with abdominal obesity persisting in girls rather than the boys6.
Throughout Europe, energy intake appears to increase during adolescence in boys, but no further increases are apparent after the age 11 years in girls7. In the only multi-centered European coordinated research, the HELENA study an inverse relationship between fat intake and BMI was demonstrated in both sexes8. Five studies have assessed the diet quality and weight status of adolescents in Greece, but with the exception of Kafatos et al9 they included wide age ranges and did not adjust the data according to age. In addition, none of the studies included blood metabolites as indicators of the sample's metabolic profile and no study has focused on a rural population.
Therefore, the need for a survey of the dietary intake of Greek adolescents was apparent. The purpose of the present cross-sectional study was twofold, to: (i) assess weight status, obesity and metabolic indices; and (ii) to record dietary intake in relation to weight status, in a rural sample of 17 year-olds.
Methods
Study population
The study took place during 2005 in the medical center of Nea Madytos, in the rural Thessaloniki prefecture, in Northern Greece. The sample was selected from the Greek mainland (ie Nea Madytos), because research has proved that in borderline areas the diet of young people is not representative10. In Greece, the term 'rural' characterizes areas with less than 10 000 inhabitants. The subjects, (n = 100) were 17 year-old students, selected randomly on one wave of recruitment from the local public schools. The inclusion criteria were willingness to participate and oral consent from a parent/guardian. Two subjects were omitted from the results due to providing insufficient data. The final sample consisted of 98 adolescents, 41 boys and 57 girls. The study was approved by the local Education Authority, the schools' headmasters and the Alexander Technological Educational Institute.
Anthropometric and biochemical indices
The body weight (BW), stature and waist circumference of participants were measured by an experienced medical doctor. Body mass index (BMI) was calculated and the international cut-off points were used in order to define underweight, normal BW or OW/OB, in accordance with Cole et al11,12. Abdominal obesity was defined as waist circumference above the 90th percentile for age and sex13.
Fasting blood samples were obtained from all subjects for the measurement of glucose and selected lipids levels including total cholesterol (TC), high density lipoprotein (HDL) and triglycerides (TG) concentrations. Impaired fasting glucose (pre-diabetes) was determined as glucose 6.1-6.9 mmol/L. Dyslipidemia was defined as TG above the 95th percentile and low HDL was defined as that below the <5th percentile for age and sex14.
Dietary intake and energy-expenditure-related lifestyle characteristics
Two previous-day recalls were attained from each participant through personal interview with a dietician. Mean nutrient intake of the recorded 2 days was analyzed with Food Processor 7.4 (ESHA; Portland, Oregon, USA), with the addition of Greek recipes. According to the Institute of Medicine guidelines15, participants were divided into low, adequate or high consumers for each macronutrient. For 17 year-old adolescents, the suggested range for adequate intake of macronutrients in relation to the energy consumption is 25-34.9% for fat, 45-64.9% for carbohydrates and 10-29.9% for proteins. With respect to these recommendations, intakes below these ranges were considered inadequate (low) and intakes greater than the proposed range indicated over-consumption (high).
Any usual involvement in sporting activities was recorded as total exercising hours per week. In accordance with these results, participants were classified as being involved in light, moderate or vigorous physical activity. These data were processed using the Institute of Medicine energy expenditure formulas for adolescents15, and the energy expenditure of each participant was calculated. The ratio of energy intake to energy expenditure (EI:EE) was calculated to define under-reporting in the energy intake according to the Livingstone and Black criteria16.
Statistical analysis
Normality of distribution was evaluated using the Kolmogorov-Smirnov test. Analysis of variance was with Bonferroni corrections, McNemar and Kruskal-Wallis tests were used to assess differences between underweight, normal BW and OW participants. The statistical software SPSS 15.0 (SPSS Inc; Chicago, IL, USA) was used for the analysis.
Results
The distribution of metabolic parameters in the study's sample is provided (Table 1). The prevalence of underweight in the total sample was 7.1%; however OW was demonstrated in 31.6% of the adolescents. Between the sexes, 4.9% of the boys and 8.8% of the girls were classified as underweight. Being a girl increased the odds for underweight (OR:1.250, CI:0.8-2.1). Approximately half the boys (51.2%) and 21.3% of the girls were OW/OB. Abdominal obesity was identified in 7.1% of the total sample (9.8% of the boys and 5.3% of the girls). Adolescent boys had increased odds for developing central obesity by 1.405 (CI:0.7-2.8), impaired fasting glucose (IFG; OR:1.200, CI:0.3-4.9), elevated triglycerides (OR:1.514, CI:1.0-2.4) and serum cholesterol concentration (OR:1.806, CI:1.1-3.1). Abdominal obesity increased the chances for IFG 8-fold (OR:8.000, CI:1.6-39.1), as well as for high triglycerides (OR:2.190, CI:0.5-9.1) and serum cholesterol levels (OR:2.167, OR:0.3-15.6).
Anthropometric and biochemical indices of the sample are presented (Table 2). Higher waist circumference and BMI were recorded in the OW participants of both sexes compared with those who were not OW (p≤0.001). No differences were observed in the median recorded metabolic analyses among the weight categories.
The nutrient intake of the sample is provided (Table 3). Phenotypically healthy boys consumed significantly more energy (KJ/kg of BW) and were better energy reporters, as indicated by the higher ratio of EI:EE compared with those who were OW (p ≤0.001). In terms of macronutrient intake, boys of normal BW demonstrated higher protein and fat intake expressed in g/kg of BW (p ≤0.01 and p ≤0.001, respectively) and increased saturated fat intake (p ≤0.05) than those who were OW. In comparison with the underweight boys, those who were OW/OB consumed less fat (p ≤0.05).
In girls, the trends in nutrient intake were different. Underweight and normal BW girls reported consuming more energy compared with those who were OW/OB (p ≤0.05). Protein intake (g/kg of BW) was more adequate in the underweight group compared with the other weight categories (p ≤0.01 for both) and carbohydrate intake (g/kg of BW) was higher than was recorded by the OW/OB group (p ≤0.05). Phenotypically healthy girls consumed more fat (g/kg of BW) and protein (%EI) compared with those who were OW/OB (p ≤0.01).
The adequacy of intake for each macronutrient is described according to the weight status of participants (Table 4). Irrespectively of their weight status, the majority of adolescents consumed a high fat and carbohydrate diet (65.3% and 93.9%, respectively), whereas protein was consumed within the proposed range by 78.6% of the participants.
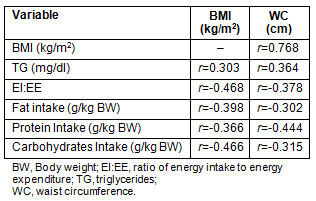
Cross-correlates of BMI and waist circumference for the total sample are presented (Table 5). The BMI and waist perimeter were highly correlated (r = 0.768, p ≤0.001), and they also demonstrated similar patterns of correlation to the triglycerides concentration, and inverse relationships with the ratio EI:EE and the reported macronutrient intake.
Table 1: Frequencies of metabolic parameters between sexes
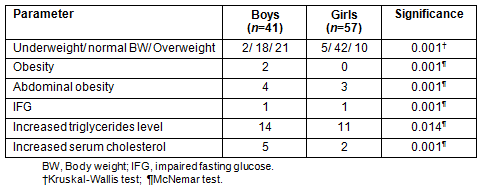
Table 2: Anthropometric and biochemical indices of participants (n = 41)
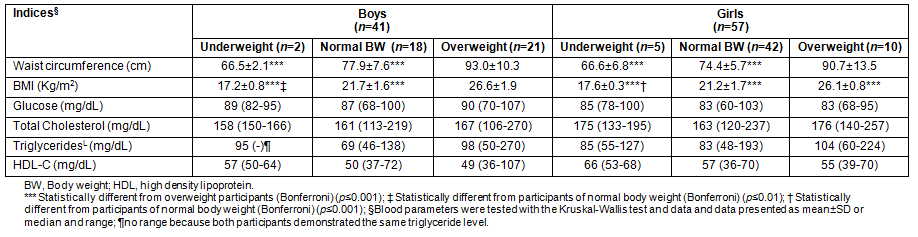
Table 3: Reported energy and selected macronutrient intake of the sample (n = 93)
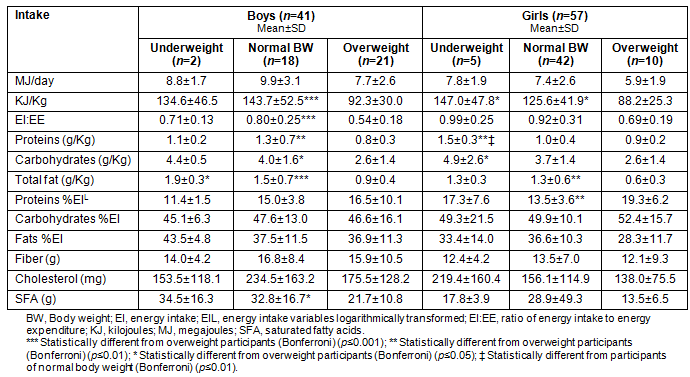
Table 4: Adequacy in the consumption of macronutrients per weight category in the total sample
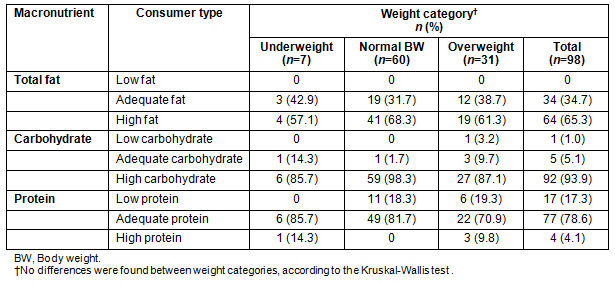
Table 5: Cross-correlates of BMI and waist circumference (p ≤0.001)
Discussion
The study showed a high prevalence of OW in 17 year-old boys in the rural Thessaloniki area (51.2%) and a lower prevalence in the girls (21.3%). Underweight was found in 7.1% of the total sample; 7.1% of the participants, the majority boys, were diagnosed with central obesity. Boys had a greater chance of having abdominal obesity, IFG, dyslipidemia and elevated serum cholesterol levels. Central obesity increased the chances of IFG (eight-fold) and doubled the prevalence of dyslipidemia and elevated serum cholesterol. Under-reporting of energy was recorded in both sexes among the OW participants and was further verified by an inverse relationship between BMI and the ratio EI:EE. Overall, the sample demonstrated a dietary pattern that was high in fats in lieu of protein.
Prevalence of overweight/underweight and central obesity
A decade ago, the prevalence of OW/OB of Northern European adolescents was reported as between 15% and 25%17. Studies in Canada and the USA demonstrated a higher prevalence of obesity among adolescents living in rural settings18,19, but research in Poland and New Zealand provided contradictory results20,21. It is as yet unclear whether rural-urban differences exist in adolescent obesity, mainly due to differences in the physical and social environment of each study's location21, as well differing definitions of urban/rural in each country. The present study classified 21.3% of the girls as OW/OB, a finding in accordance to previous research on the prefecture of Thessaloniki5, but relatively high, compared with the whole of Greece (16.7%)6. Among the participating boys, approximately half were OW/OB (51.2%), a proportion exceeding the prevalence suggested by previous research in the country5,6. Similar findings have been made in South Carolina, where almost 50% of the rural sample was classified as obese, more than double the national average22. Although Tzotzas et al included rural residents in their study, as in the present research they failed to demonstrate differences in the prevalence of adolescent OW among urban, rural and semirural areas of Greece6. Similar research in the Spanish county of Aragón also failed to demonstrate rural-urban differences in overweight23. However, both studies included samples spanning in a wide age range; whereas, in the present study, only 17 year-olds were recruited. Thus, the attenuated prevalence of OW in the boys in the Tzotzas et al study may be the result of a birth-cohort effect6. According to Olsen, the trends in prevalence of obesity must be expressed by year of birth, rather than as a wide-ranging age population24. In this way certain environmental factors to which the cohorts were exposed that may have altered their weight status (advertising, economic crisis, trends in body image, epidemic etc) are accounted for. Thus, although the prevalence of OW in the present study's boys is higher than in previous research, it may actually imply a birth cohort effect of an 'obesogenic' nature, to which the adolescents of Nea Madytos were exposed.
Overall, 4.9% of the boys and none of the girls were classified as OB. A recent study examining the prevalence of obesity in children aged 4-11 years, all inhabitants of Nea Madytos25, demonstrated an increasing tendency for obesity that peaks at the age of 9 years and declines afterwards. At the age of 11 years (the oldest age group examined), 12.1% of the boys and 13.6% of the girls were classified as OB according to the International Obesity Task Force criteria. Therefore, it is logical to expect even lower rates of obesity in 17 year-old adolescents from Nea Madytos, which in conjunction with the high OW rate indicates that the small proportion of obesity is actually the result of accelerated growth and not a better quality diet.
The present study was the first to examine the prevalence of thinness in Greek adolescents. Even internationally, research using international cut offs for underweight is scarce. Among the few studies retrieved, a recent survey based in Tuscany demonstrated a similar prevalence of thinness among 15 year-old Italians (8.7%)26. In Germany, 12.6% of teenage boys and 19.1% of teenage girls were underweight27; however, because a wide age range was included in the sample (11-17 years) it is possible that the high prevalence of underweight may have resulted from the accelerated growth that takes place during the first stages of puberty. In Portugal, underweight was found to increase with age throughout adolescence; among 18 year-olds 7.6% of girls and 7.3% of boys were reported to be underweight28.
Abdominal obesity was diagnosed in 7.1% of the sample, and coincided either with OW or obesity. In the 17-19 years group of the Greek nationwide study the prevalence of central obesity was 9.9% for boys and 15.6% for girls6. The present study demonstrated a similar trend in boys, but among girls only 5.3% were diagnosed with abdominal obesity. The lower proportion of abdominal obesity in the present study's girls and the greater proportion of OW/OB compared with the nationwide study implies that in adolescent girls of Nea Madytos, excess in body weight is not necessarily accumulated on the waist area, but possibly on the hips as well, as is often seen in Mediterranean countries29. According to the present data, an accumulation of visceral fat increases the chances for IFG (eight-fold) and doubles the prevalence of dyslipidemia. The International Diabetes Foundation postulates abdominal obesity as the sine qua non of the metabolic syndrome30, a clustering of obesity, diabetes, hypertension, dyslipidemia and low-HDL, which attenuates the risk for developing cardiovascular disease. The good correlation between the triglycerides level and both BMI and waist circumference demonstrated in the present study verifies the relationship between dyslipidemia and obesity.
Dietary intake
The majority of participants under-reported energy intake. Under-reporting energy has also been demonstrated in adolescents of normal body weight31. Livingstone and Black reviewed a series of studies based on adolescents and concluded that reporting of energy intake is generally poor among teenagers, with the ratio EI:EE being 0.81 ± 0.14, a number akin to that proposed by the present findings (0.79 ± 0.30)16. The low ratio of EI:EE during adolescence has been attributed to increased energy requirements, a pattern of unstructured eating, concerns with self-image and rebellion against authority16. Participants with normal BW exhibited a mean fat intake (expressed as a percentage of the energy intake) beyond the recommended 35% for adolescents15. This dietary pattern that is characterized by large amounts of total fat and saturated fatty acids has been reported to prevail in Spanish and Greek adolescents5,17. Lambert et al suggested the existence of a homogenous trend in cholesterol consumption among adolescents in Europe7. However, the fiber intake of the sample was inadequate because it failed to exceed the proposed adequate intake of 38 g for boys and 26 g for girls15. Overall, the present sample demonstrated a dietary pattern high in fats and low in protein, a finding in accordance to previous research in Thessaloniki5. This pattern is indicative of the abandonment of the traditional diet in favor of one more westernized and has been demonstrated among the Greek young people throughout the country5,10,32. It is, however, alarming that this obesogenic dietary pattern was not only demonstrated by the OW participants, but persisted in the total sample. Research in Cyprus failed to show major differences between the diets of urban and rural young people; however, the latter tended to consume more traditional foods and were less likely to eat 'fast foods'33. This was further verified by Lachat et al, who demonstrated that during adolescence, living in an urban area is correlated with more frequent eating outside the home34. Research on a rural-urban Greek sample showed that OW adolescents received significantly more pocket money35, and that the amount of pocket money was correlated to the consumption of energy, dietary fat, saturated fat, and sugars. According to these studies, it is highly possible that the adoption of a low quality diet among the present sample is the result of eating outside the home and spending pocket money on unhealthy foods, factors not included in the study design.
Serum lipids and impaired fasting glucose
The serum cholesterol levels of adolescents in Northern Europe have increased in the last decades17. Literature has shown a relatively weak relationship between dietary intake and blood analysis7 and in the present study no correlation was established between the reported intake of cholesterol and the serum cholesterol concentration. The present teenagers demonstrated similar serum cholesterol levels with OB adolescents of Northern Greece36. In parallel, dyslipidemia was diagnosed in 25.5% of the sample, and abdominal obesity doubled the odds for co-morbid dyslipidemia.
An IFG was identified in 2.0% of the sample, a finding similar to the results of the Third National Health and Nutrition Examination Survey (NHANES III)37. According to the present data, adolescents with central obesity have attenuated odds (eight-fold) for developing IFG. A recent study on a sample of Greek young people with 'diabesity' (ie obesity and type 2 diabetes/pre-diabetes) suggested that 'diabese' children and adolescents have a four-fold greater chance of developing metabolic syndrome36. The study also linked the syndrome to inflammatory markers, representing evidence of early adverse effects on the cardiovascular system of the adolescents. As IFG detected in the present study is a strong risk factor for diabetes mellitus and metabolic syndrome, detection of IFG must be pursued by any clinical approach directed at preventing diabetes mellitus and cardiovascular disease.
Limitations
The present study, however, is not without caveats. The sample, although homogenous, was relatively too small to draw general conclusions. In parallel, the lack of a control group from the metropolitan area of Thessaloniki limited the strength of the results. However, arterial blood pressure, if measured, would have provided the data needed to assess the prevalence of metabolic syndrome. Future research with a longitudinal design is needed in order to identify whether the unhealthy dietary patterns observed in adolescents are simply evidence of adolescent risk-taking behavior or a continuation of unhealthy eating that started during childhood, as has been exhibited in a great majority of studies of Greek children5,10,38,39. However, the limited existing literature increases the need for research into adolescent diet. Very few studies assessing diet during adolescence have included blood analysis in their methodology7, which is an advantage of the present study. The fact that the sample consisted of a small cohort is another advantage, because the majority of dietary surveys on adolescents lack consistency of age or age cut-off points among participants surveyed7.
Conclusions
With adolescent health one of the priorities of the WHO agenda, these results indicate the need for educating adolescents on health issues. Proper nutrition is basic to disease prevention. Because adolescent health spans a continuum from childhood to adult health, nutrition during adolescence should be a research priority for preventive medicine. The majority of teenagers engage in risk-taking and unhealthy behaviors; however, research has shown that education in an adolescent-friendly form may motivate the teenagers and ameliorate their unhealthy eating practices40.
The present study is the first to examine weight status and diet in a rural sample of Greek adolescents. The findings demonstrated a high prevalence of OW among 17 year-old boys of rural residence. Among boys the prevalence of OW/OB was approximately double that of the national average, indicating that preventing adolescent OW should be a priority for rural policy. As far as obesity is concerned, the present data, in conjunction with recent data on children of Nea Madytos, demonstrate that the low proportion of obesity in 17 year-old adolescents of rural residence is more likely to be the result of accelerated growth and not a healthy diet. Underweight was demonstrated in 7.1% of the sample. Central obesity was more frequent in boys compared with girls and increased the odds for demonstrating IFG and dyslipidemia. Fewer participating girls were diagnosed with central obesity compared with the nationwide study, indicating that weight deposition does not occur in the abdominal area, as has been observed in other studies on Mediterranean women. Overall, a more energy- and nutrient-dense diet was observed in the participants who were underweight and of normal body weight, compared with those who were OW. Data on dietary intake showed that irrespectively of their weight status, adolescents consume a diet high in fats in lieu of protein. Thus, dietary counseling, as a means of preventive medicine, should be available for all adolescents, irrespectively of their weight status.
References
1. World Health Organization. Adolescent friendly health services. An agenda for change. WHO, 2002. (Online) 2002. Available: http://whqlibdoc.who.int/hq/2003/WHO_FCH_CAH_02.14.pdf (Accessed 23 November 2010).
2. Golden BE. Infancy, childhood and adolescence. In: JS Garrow, WPT James, A Ralph (Eds). Human Nutrition and Dietetics. Edinburgh: Churchill Livingstone, 2000; 449-464.
3. Margetts B. Are we paying enough attention to adolescent nutrition? Public Health Nutrition 2009; 12: 145-146.
4. Wang Y, Lobstein T. Worldwide trends in childhood overweight and obesity. International Journal Pediatric Obesity 2006; 1: 11-25.
5. Hassapidou M, Fotiadou E, Maglara E, Papadopoulou SK. Energy Intake, Diet Composition, Energy Expenditure, and Body Fatness of Adolescents in Northern Greece. Obesity 2006; 14: 855-862.
6. Tzotzas T, Kapantais E, Tziomalos K, Ioannidis I, Mortoglou A, Bakatselos S et al. Epidemiological Survey for the prevalence of overweight and abdominal obesity in Greek adolescents. Obesity 2008; 16: 1718-1722.
7. Lambert J, Agostoni C, Elmadfa I, Hulshof K, Krause E, Livingstone B et al. Dietary intake and nutritional status of children and adolescents in Europe. British Journal of Nutrition 2004; 92: S147-S211.
8. Kersting M, Sichert-Hellert W, Vereecken CA, Diehl J, Béghin L, De Henauw S et al. Food and nutrient intake, nutritional knowledge and diet-related attitudes in European adolescents. International Journal of Obesity 2008; 32: S35-S41.
9. Kafatos A, Verhagen H, Moschandreas J, Apostolaki I, Van Westeropp JJ. Mediterranean diet of Crete: foods and nutrient content. Journal of the American Dietetic Association 2000; 100: 1487-1493.
10. Grammatikopoulou MG, Daskalou E, Hatzopoulou M, Sourtzinou L, Tsigga M. Comparing diet composition and growth of children living in two limitary Greek islands (Samos and Corfu). Public Health Nutrition 2009; 12: 1284-1289.
11. Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ 2000; 320: 1240.
12. Cole TJ, Flegal KM, Nicholls D, Jackson AA. Body mass index cut offs to define thinness in children and adolescents: international survey. BMJ 2007; 335: 194-202.
13. Fernandez JR, Redden D, Pietrobelli A, Allison DB. Waist circumference percentiles in nationally representative samples of African-American, European-American, and Mexican-American children and adolescents. Journal of Pediatrics 2004; 145: 439-444.
14. Schulpis K, Karikas GA. Serum Cholesterol and Triglyceride distribution in 7767 school-aged Greek children. Pediatrics 1998; 101: 861-864.
15. Institute of Medicine. Dietary Reference Intakes. Washington DC: National Academies Press, 2005.
16. Livingstone MB, Black AE. Markers of the Validity of Reported Energy Intake. Journal of Nutrition 2003; 133: 895S-920S.
17. Cruz JA. Dietary habits and nutritional status in adolescents over Europe-Southern Europe. European Journal of Clinical Nutrition 2000; 54: S29-S35.
18. Joens-Matre RR, Welk GJ, Calabro MA, Russell DW, Nicklay E, Hensley LD. Rural-urban differences in physical activity, physical fitness, and overweight prevalence of children. Journal of Rural Health 2008; 24(1): 49-54.
19. Bruner MW, Lawson J, Pickett W, Boyce W, Janssen I. Rural Canadian adolescents are more likely to be obese compared with urban adolescents. International Journal of Pediatric Obesity 2008; 3: 205-211.
20. Suliga E. Socio-economic differentiation of the growth and the dietary intake of Polish boys aged 7-16 years. Annals of Human Biology 2009; 36: 199-210.
21. Hodgkin E, Hamlin MJ, Ross JJ, Peters F. Obesity, energy intake and physical activity in rural and urban New Zealand children. Rural and Remote Health 10(2):1336. (Online) 2010. Available: http://rrh.org.au (Accessed 23 November 2010).
22. Tai-Seale T, Chandler C. Nutrition and overweight concerns in rural areas: a literature review. Rural Healthy People 2010: A companion document to Healthy People 2010. Volume 2. College Station, TX: The Texas A&M University System Health Science Center, School of Rural Public Health, Southwest Rural Health Research Center, 2003. Available: http://srph.tamhsc.edu/centers/rhp2010/09Volume1nutrition%20.htm (Accessed 23 November 2010).
23. Ara I, Moreno LA, Leiva MT, Gutin B, Casajús JA. Adiposity, physical activity, and physical fitness among children from Aragón, Spain. Obesity 2007; 15(8): 1918-1924.
24. Olsen LW, Baker JL, Holst C, Sørrensen TI. Birth cohort effect on the obesity epidemic in Denmark. Epidemiology 2006; 17: 292-295.
25. Mavrakanas TA, Konsoula G, Patsonis I, Merkouris BP. Childhood obesity and elevated blood pressure in a rural population of northern Greece. Rural and Remote Health 9:1150. (Online) 2009. Available: http://rrh.org.au (Accessed 23 November 2010).
26. Lazzeri G, Rossi S, Pammolli A, Pilato V, Pozzi T, Giacchi MV. Underweight and overweight among children and adolescents in Tuscany (Italy). Prevalence and short-term trends. Journal of Preventive Medicine & Hygiene 2008; 49: 13-21.
27. Mikolajczyk RT, Richter M. Associations of behavioural, psychosocial and socioeconomic factors with over- and underweight among German adolescents. International Journal of Public Health 2008; 53: 214-220.
28. Marques-Vidal P, Ferreira R, Oliveira JM, Paccaud F. Is thinness more prevalent than obesity in Portuguese adolescents? Clinical Nutrition 2008; 27: 531-536.
29. Grammatikopoulou MG, Papadopoulou SK, Zakas A, Mylona A, Kapsalis I. Dietary intake of free-living elderly in northern Greece. Journal of Nutrition for the Elderly 2006; 26: 131-146.
30. International Diabetes Federation. The IDF consensus definition of the metabolic syndrome in children and adolescents. Brussels: International Diabetes Federation, 2007.
31. Bratteby LE, Sandhagen B, Fan H, Enghardt H, Samuelson G. Total energy expenditure and physical activity as assessed by the doubly labeled water method in Swedish adolescents in whom energy intake was underestimated by 7-d diet records. American Journal of Clinical Nutrition 1998; 67: 905-911.
32. Van Diepen S, Scholten AM, Korobili C, Kyrli D, Tsigga M, Van Dieijen T et al. Greater Mediterranean diet adherence is observed in Dutch compared with Greek university students. Nutrition, Metabolism & Cardiovascular Diseases 2009; (in press), DOI:10.1016/j.numecd.2009.11.006.
33. Lazarou C, Kalavana T. Urbanization influences dietary habits of Cypriot children: the CYKIDS study. International Journal of Public Health 2009; 54: 69-77.
34. Lachat C, Khanh le NB, Khan NC, Dung NQ, Nguyen do VA, Roberfroid D et al. Eating out of home in Vietnamese adolescents: socioeconomic factors and dietary associations. American Journal of Clinical Nutrition 2009; 90: 1648-1655.
35. Grammatikopoulou MG, Galli-Tsinopoulou A, Daskalou E, Tsigga M, Stylianou C, Kokka P et al. Is pocket-money an indicant of dietary intake and obesity? Archives of Disease in Childhood 2008; 93: 4.
36. Galli-Tsinopoulou A, Grammatikopoulou MG, Stylianou C, Emmanouilidou E, Kokka P. Diabese youngsters have 3.7 more chances in developing metabolic syndrome compared with the obese. Journal of Endocrinological Investigator 2010; 33: 549-553.
37. Muntner P, He J, Chen J, Fonseca V, Whelton PK. Prevalence of non-traditional cardiovascular disease risk factors among persons with impaired fasting glucose, impaired glucose tolerance, diabetes, and the metabolic syndrome: analysis of the Third National Health and Nutrition Examination Survey (NHANES III). Annals of Epidemiology 2004; 14: 686-695.
38. Angelopoulos P, Kourlaba G, Kondaki K, Fragiadakis GA, Manios Y. Assessing children's diet quality in Crete based on Healthy Eating Index: the Children Study. European Journal of Clinical Nutrition 2009; 63: 964-969.
39. Papadaki A, Linardakis M, Codrington C, Kafatos A. Nutritional intake of children and adolescents with insulin-dependent diabetes mellitus in crete, Greece. A case-control study. Annals of Nutrition and Metabolism 2008; 52: 308-314.
40. Olson AL, Gaffney CA, Lee PW, Starr P. Changing adolescent health behaviors. The Healthy Teens Counseling Approach. American Journal of Preventive Medicine 2008; 35: S359-S364.
Abstract
Introduction:
With adolescent health a priority on the WHO agenda, research into the diet, weight status and metabolic profile of adolescents is indicated. The present study aimed to assess the diet and metabolic parameters of a rural sample of adolescents.Methods: One hundred adolescents (17 years of age) were recruited from schools in Nea Madytos, Thessaloniki, Greece. Two previous-day food recalls were collected for each participant, and weight, height, waist circumference, serum lipids and fasting glucose levels were measured. The prevalence of underweight/overweight, central obesity, dyslipidemia and impaired fasting glucose (IFG) were calculated.
Results: Overweight was present in half the boys (51.2%) and one-fifth of the girls (21.3%). In the total sample 7.1% were underweight and another 7.1% were diagnosed with central obesity. Boys had an increased risk of abdominal obesity (OR:1.405, CI:0.7-2.8), IFG (OR:1.200, CI:0.3-4.9) and elevated triglycerides (OR:1.514, CI:1.0-2.4) and serum cholesterol levels (OR:1.806, CI:1.1-3.1). Central obesity increased the chances of IFG (OR:8.000, CI:1.6-39.1) and doubled the prevalence of dyslipidemia (OR:2.190, CI:0.5-9.1). Under-reporting of energy was found among overweight participants and was further verified by an inverse relationship between BMI and the ratio of energy intake to energy expenditure. Adolescents identified a dietary pattern high in fats in lieu of protein.
Conclusions: Irrespectively of their weight status, teenagers consume a high fat diet; therefore, dietary counseling, as a means of preventive medicine, should be applied to all weight categories. In addition, the prevalence of obesity in a rural sample of adolescents appears to be higher compared with the whole of Greece.
Key words: diet, Europe, impaired fasting glucose, nutrition, obesity, overweight, prediabetes, teenagers, underweight.
You might also be interested in:
2016 - The answers are out there! Developing an inclusive approach to collaboration
2006 - Health and information in Africa: the role of the journal Rural and Remote Health
