Introduction
Colorectal cancer (CRC) is the third most commonly diagnosed cancer and the second leading cause of cancer deaths in both men and women in the USA1. It is one of the few cancers that can be prevented through screening, and if detected at an early, localized stage, the relative five-year survival is 91%1. The CRC screening modalities for the average-risk population fall into two categories: (i) those used to detect adenomatous polyps and cancer (eg flexible sigmoidoscopy, barium enema examination, computed tomographic colonography [CTC] or 'virtual colonoscopy' , and colonoscopy); and (ii) those used primarily to detect cancer (eg annual fecal occult blood test [FOBT], annual fecal immunochemical test, and stool DNA test, performed at uncertain intervals). Asymptomatic adults aged 50 years and older are recommended to have colonoscopy and CTC every 10 years, and all other tests that detect adenomatous polyps and cancer every 5 years2.
Although in the past several years, much progress has been made in reducing CRC incidence and mortality, efforts need to be intensified to increase availability and utilization of CRC screening particularly in areas where CRC rates remain to be elevated. More specifically, in Kentucky, CRC is the second leading cause of cancer death, with CRC mortality in Appalachian Kentucky 11% higher than national average3.
It is unclear why CRC rates are elevated in Appalachian Kentucky. However, modifiable risk factors for CRC that include low socioeconomic status (SES), tobacco use, and obesity, are relatively common in this region1. Appalachian Kentuckians have twice the poverty rate of their national counterparts, significantly higher rates of smoking (29% compared with 25%), and the ninth highest rate of obesity in the USA4.
In addition, CRC screening, a key tool in early detection, prevention, and treatment of CRC, may be underutilized in Appalachian Kentucky5,6. In 2002, the CRC screening rate among those aged 50 years and older was 38% in Appalachian Kentucky (vs 44% in Kentucky and 49% in the USA)5,7.
The purpose of the present study was to explore the uptake or prevalence of and explanations for CRC screening among an especially vulnerable group of older adults, those of lower SES, living with multiple morbidity (MM), and residing in a rural location (Appalachian Kentucky). Currently, there is a paucity of research on best practices for caring for people with MM, and care for chronic morbidities is skewed toward utilizing research on single conditions8. Furthermore, the relationship between MM and the uptake of preventing future morbidities through cancer screening in particular, remains unresolved. Since patients with MM make more frequent visits to physicians' offices9-12, they may have more opportunities to receive a physician's recommendation for screening, a major contributor to obtaining CRC screening13-15. Alternatively, because MM is frequently mentioned as a barrier to screening, and also Appalachian and other rural, traditionally underserved populations are known to experience a more pernicious version of the nation's health problems, a higher priority for diagnosed conditions rather than preventive health behaviors might be anticipated.
Methods
The data for this study came from the first phase of a three-part study conducted from May 2008 to April 2010, which was designed to address whether and how the increasingly common phenomenon of MM affects the receipt of cancer screenings in a health disparity population. This project used an integrated and simultaneous mixed methods approach using the qualitative, open-ended questions and the quantitative instruments, health, and demographic data to complement each other16. During this first phase, a trained local interviewer conducted two face-to-face interviews with 41 participants having two or more chronic conditions. Interviews lasted 60-90 minutes and took place at a mutually agreed upon location. Each participant gave informed consent prior to the first interview and permission to audiotape the interviews. At the end of the first interview, the interviewer administered a socio-demographic questionnaire. Due to the limited literacy of the informants, most of the questions and structured instruments were administered orally. All research protocols were approved by the University of Kentucky Institutional Review Board.
Study location, recruitment, and interview protocol
Study location: Appalachia is a geographically and culturally diverse region of 410 counties in 13 states that contain almost 22 million people, or 8% of the US population. Appalachia has long been characterized as a region of the country with high rates of extreme poverty, isolation, and poor health6. Appalachian Kentucky (54 of 120 counties) has SES and educational indicators that are among the lowest in the USA. These indicators are highlighted (Table 1), including profiles of the four Kentucky Appalachian counties where this study took place17.
Table 1: Selected sociodemographic characteristics of the USA, Kentucky, Appalachian Kentucky, and the 4 Counties of the research17
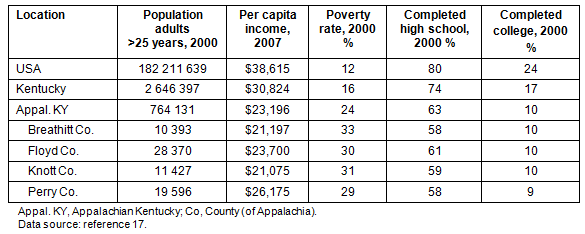
The lower SES and health indicators are exacerbated by persistent health care provider (HCP) shortages. For instance, the rate of primary care physicians in Appalachia is 56 per 100 000 in comparison with 96 per 100 000 in urban USA4. Almost half of Kentucky's counties (55 of 120), and most of them rural, are officially designated Health Professional Shortage Areas for primary care18. Although data are lacking on specialists such as gastroenterologists (GIs) in Appalachia, the USA as a whole has a shortage of GIs19. It is reasonable to expect a shortage of GIs in Appalachian Kentucky, considering that 43% of Kentucky residents as compared to 28% of Kentucky physicians live in rural areas20. One would expect that high rates of poverty, low educational attainment, persistent distressed county designation, and high levels of HCP shortages would lead to disadvantages in CRC cancer screening, particularly among those with competing medical demands.
Recruitment and interview protocol: Participants were recruited from three family and community medicine practices in Appalachian Kentucky. These practices were selected based on their willingness to engage in the research, the appropriateness of their patients (eg a general clinic, as opposed to a pediatric specialty clinic), and their location in counties with fairly representative characteristics for Appalachian and other rural underserved populations.
Given the researchers' interest in vulnerable, hard to reach patients, a purposive, non-random sampling approach was employed. Within each of medical practice, staff compiled a list of up to 100 patients aged 50-76 years seen in the clinic over the past year. The providers reviewed the lists and identified those patients who met the eligibility criteria (stated below). The medical practices mailed these potential participants a letter of invitation to be involved in two interviews to explore participants' health decision-making. The letter stated that those interested in participating or finding out additional information should mail back the self-addressed stamped letter. Once the letters were received, potential participants were phoned for verification and to ensure that they met the eligibility criteria. All but three individuals interested in participating were included.
Age-eligible patients with 2 or more conditions recognized as chronic and requiring fairly extensive self-care and/or formal medical managements were included21. Consistent with the National Center for Health Statistics' definition, 'chronic conditions' were conceptualized as any illness that lasts for at least 3 months22. Our investigation was limited to individuals aged 50-76 (the recommended start and end ages for CRC screening, according to the US Preventive Services Task Force [US PSTF]); a particular focus on those 50-64 years is warranted because their lack of Medicare coverage may increase vulnerability23,24.
Patients with six or more MM were excluded, a relatively rare occurrence, because there is debate about the relative benefit of screening for those with numerous illnesses. In consultation with the project physician, also excluded were those with colostomy, Crohn's Disease, iron deficiency anemia, ulcerative colitis, rectal bleeding, lower abdominal pain, and irritable bowel syndrome, all of whom may undergo endoscopy for reasons other than CRC screening. Potential participants also were excluded if they were unable or unwilling to be involved in the study or had received a diagnosis of cancer for a site in which screening could occur (ie cervical, breast, colorectal). To insure broad perspectives on CRC screening, CRC screening status was not an eligibility criterion.
On verification of eligibility, a local resident extensively trained and experienced in conducting face-to-face interviews arranged a time and location to meet participants, most often in the participants' homes. At the initial meeting, the interviewer requested that session took place in a private and quiet setting, explained the purpose of the study, and answered remaining questions. An honorarium ($25 for the first interview and $35 for the second interview) was paid on completion of each interview.
To optimize the establishment of rapport and trust, general information was asked during the first round of interviews, followed by more personal information on the second. Immediately on terminating the interview, the interviewer wrote field memos relevant to the session, including noting the presence of others, the mood of the participant, and any relevant circumstances that might influence the data retrieved. To enhance retention efforts, the second interview took place within one month of the initial interview. The interviewer phoned the day before to confirm the meeting and began the session by completing any questions left over from the previous interview. As explained in the results, 43 individuals were recruited into the project, and ultimately, 41 were eligible and retained.
Data sources and analysis
The data used in this study came from semi-structured and structured questions. The researchers' main question of interest was: 'When did you have your last colorectal cancer screening test?'
In order to address the way in which having multiple chronic conditions might shape CRC screening, the following framing questions were asked with prompts:
- Some people say that having some health conditions makes it harder to get screened for cancer. Others say it doesn't matter. What are your thoughts?
- Does the doctor recommend cancer screening?
- What about taking care of those other health concerns?
- Other thoughts?
- Can you recall a specific time when you didn't get screened for cancer? What made it hard for you?
Other questions included self-assessed health, medical conditions, and socio-demographic characteristics. The interview transcripts were also reviewed to identify and quantify the type of screening (ie modality) a participant had and whether participants were in compliance (ie adherence) with the CRC screening, as recommended by major professional medical association23.
The quantitative data from the questionnaires and transcripts were entered into Microsoft Excel 2007 and recoded, and all qualitative data were transcribed and qualitatively analyzed25. Specifically, on completion of the interviews' transcription as a review for accuracy, researchers re-read each of the transcripts, conducting line-by-line coding to compile a codebook26. As new codes emerged, they were added to and redefined the original codebook. Coding outcomes were periodically compared among the researchers to ensure consistency. Discrepancies were addressed by further modifying the codebook and recoding the transcripts. This iterative process of coding, comparing codes, clarifying instances of discrepant codes, and re-coding was repeated until an inter-coder reliability ratio of approximately 80%27 was established. To assess the differences in CRC screening with regard to socio-demographic and health status, a Kruskal-Wallis and Fisher's exact tests were performed using Stata/IC 10.0 for Windows (StataCorp LP, College Station, TX, USA).
Results
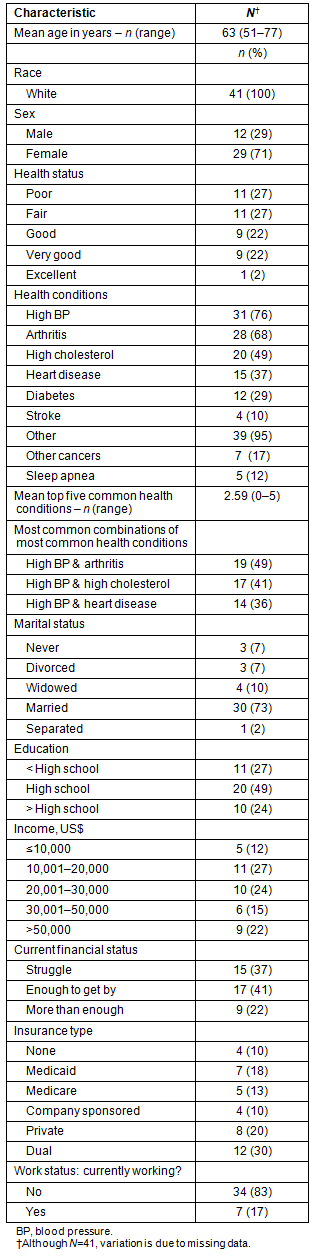
The final sample consisted of 41 participants. Of the initial 43 interviewed participants, one individual was unable to continue and withdrew after the first interview, and another belatedly reported having colon cancer and was excluded from the study. Consistent with the general population of the study area28, all of the participants were white (Table 2). The average participant's age was 63 years, ranging from 51 to 77 years. The sample predominantly consisted of participants who were female (71%) and married (73%). More than half (51%) of the sample had annual incomes between $10,001 and $30,000, and only 22% characterized their financial status as 'more than enough' . Fewer than one-quarter of participants (24%) had more than a high school education. Ten percent had no health insurance coverage; others had Medicaid (18%), Medicare (13%), company-sponsored (10%), private (20%), or dual (30%) insurance coverage.
The participants predominantly reported their health status as either poor (27%) or fair (27%), with only one participant reporting excellent health. The most commonly reported health conditions were high blood pressure (76%), arthritis (68%), high cholesterol (49%), heart disease (37%), and diabetes (29%). The average number of chronic conditions reported was 3 (range 2-5). The most common combination of MM was arthritis and high blood pressure followed by high blood pressure and high cholesterol, and high blood pressure and heart disease.
Table 2: Socio-demographic and health-related characteristics of the sample
Prevalence of colorectal cancer screenings
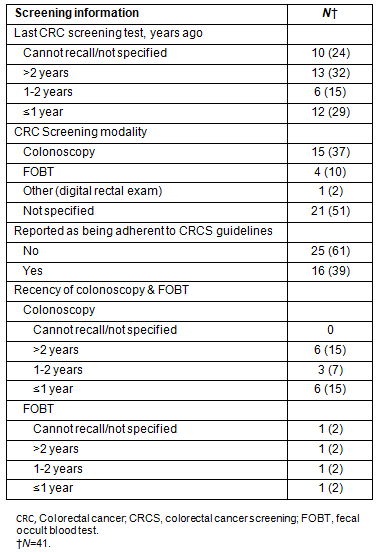
Fewer than half of the participants (44%) reported having had their last CRC test one to 2 years ago, and almost one-third (32%) indicated that they had experienced a CRC screening test over 2 years ago. Twenty-four percent of the sample could not recall if and when they had their last CRC screening test. Of those indicating receipt of a CRC screening, 37% indicated they had a colonoscopy, including virtual colonoscopy; 10% reported receiving FOBT, and 2% indicated a digital rectal examination; whereas 51% could not recall the CRC screening modality. Thirty-nine percent of the total sample received their screenings in accordance with medical guidelines23, including one participant undergoing FOBT and 15 participants (37%) having a colonoscopy. Information is provided on CRC screening, including self-reported adherence and modalities (Table 3).
Table 3: Colorectal cancer screening information
Factors Related to colorectal cancer screening patterns
To examine possible predictors of CRC screening, the relationship between date of last screening, adherence to guidelines, screening modalities, and socio-demographic data including self-rated health status and health conditions, respectively, were quantitatively analyzed. A statistically significant relationship was found between the date of last screening and adherence to CRC screening guidelines (p = 0.007). No statistically significant associations were found between the date of last screening and socio-demographic sample characteristics. Similarly, no statistically significant associations were found between adherence to guidelines and socio-demographic characteristics, nor between screening modalities and socio-demographic characteristics.
Transcript analysis revealed barriers and facilitators of CRC screening roughly corresponding to patient, medical system, and community factors. Of particular relevance, participants described that MM might shape screening decisions in one of three ways: (i) MM plays little or no role in the uptake of screening; (ii) MM presents a barrier to screening; and (iii) MM enhances the likelihood of screening. Those individuals who described MM as a barrier to screening provided the following explanations:
- Prevention was a secondary concern compared to disease management.
- Preparation necessary for colonoscopy might interfere with disease management, particularly in the case of diabetes or other conditions requiring medication.
- Physical limitations, including mobility concerns, might make screening preparation difficult.
- Inadequate finances force people to choose disease management (which they know was necessary for their survival) or prevention (which seemed hypothetical and less urgent).
Additionally, participants articulated 'illness fatigue' - or the sense of being tired of spending time and money to undergo unpleasant procedures rather than just living their lives. The far fewer individuals who mentioned that MM facilitated screening noted that doctors could do those screening tests while they checked on patients' existing conditions, and since those individuals had insurance for their other conditions, they could use such cover to finance cancer screening tests. No patterns emerged on the screening status of those expressing these perspectives.
Discussion
This research explored the prevalence of and explanations for CRC screening among an especially vulnerable group of adults - those of lower SES, living with MM, and residing in Appalachia. Only 39% of the sample reported receiving a CRC screening consistent with medical guidelines23; all but one (ie FOBT) of these screenings were colonoscopy. Most of those receiving FOBT were out of compliance. Only 24% could not recall if and specify when they had their last CRC screening test; however, slightly more than half (51%) could not recall their screening modality. It is likely that owing to the modest sample size, no socio-demographic and health-related predictors of screening emerged.
Given the challenging socioeconomic and environmental circumstances facing this sample, these modest rates of screening might be expected. Additionally, these data conform to Behavioral Risk Factor Surveillance System data on CRC screening in the Appalachian region7. However, the results raise concerns for several reasons. First, 39% may be an overestimation of adherence given: (i) the tendency of people to think they have had a test more recently than they actually did29; and (ii) the high prevalence of CRC in the region that increases the possibility that some of the participants may need more frequent screenings. Thus, it is speculated that the more frequent screening by some participants obscure the inadequate screenings of others. This modest level of screening is of particular concern given that the participants are the ones who visit their physicians on a fairly regular basis. Furthermore, the continued use of digital rectal examinations - no longer considered an acceptable CRC screening modality - raises worries about the quality of medical care.
Additionally, since CRC rates are elevated in the Appalachian region and several CRC risk factors are common in close kin networks (family history, smoking and eating patterns, likelihood of getting screened), many of the participants should be considered high priority populations for CRC screening. According to 20% of participants, their physician had recommended more frequent screenings owing to family history or other risk factors that elevate their susceptibility to CRC. These individuals reported screenings within standard medical guidelines, but would likely fall outside of the guidelines if they were considered high risk.
Finally, it is concerning that slightly more than half of the participants were unable to specify the CRC screening modality; and that fewer than one-fourth of the participants could not recall or specify the date or time period when they had their last CRC screening test. The source of uncertainty is unclear. Perhaps, with such frequent physician visits, participants have become accustomed to their providers arranging their medical care and do not remain vigilant about preventive screenings. Alternatively, since CRC screenings are among the newer preventive health measures, there may be a continuing lack of awareness of the screening recommendations. Additionally, in the context of many other competing health demands, participants and their physicians may simply de-prioritize this preventive behavior30.
Our initial speculation - that because all of the participants had MM, they might have made more frequent visits to physicians' offices, have a medical home, and have had several specialist physicians, and thereby enjoy a greater 'window of opportunity' for screenings to be recommended and occur - was not confirmed either quantitatively or qualitatively. Although several participants mentioned having more opportunities to receive a screening recommendation - a major contributor to obtaining CRC screening12-14 - they appear to lag behind state and national screening rates31. Additionally, as the narrative data suggest, competing resource demands, higher priority given to existing conditions, and concern about physical frailty might have decreased the likelihood of CRC screening in this multi-morbid, low income rural population30.
The study is limited by the use of purposive sampling technique and self-reported data. Additionally, since we aimed to benefit from the richness of a mixed methods design, the sample size was modest. Thus, readers are cautioned against using these findings beyond the intended purposes of exploring CRC screening rates and explanation among a vulnerable population in Appalachian Kentucky. Nevertheless, as the participants are considered hard-to-reach, the use of non-probability sampling technique was justifiable.
Conclusion
Although multiple morbidity status offers great challenges to disease prevention activities, the authors remain persuaded by participants who suggest that the window of opportunity left open by more frequent visits to the HCP should be used to patients' advantage. This is especially true as people with MM are living longer with their chronic conditions, particularly given the decreasing age for chronic conditions. Efforts must accelerate to establish clinical guidelines and practices that incorporate both management of existing and prevention of future conditions.
Acknowledgements
Support for the research was provided by the National Cancer Institute/National Institutes of Health through the grant, 'Increasing Colorectal Cancer Screening for Patients with Multiple Morbidities' (PI: Schoenberg), R21 CA129881. The project was approved by the University of Kentucky Institutional Review Board (#07-0843-P2H). The authors acknowledge and thank Drs Casey, Moore, Pearce, and Sloan for their assistance with physician insights and participant recruitment. They also express their appreciation of Ms Charlotte Davis, Dr Steven Fleming, and the anonymous reviewers. Last but not least, the authors thank the participants.
References
1. American Cancer Society. Cancer Prevention & Early Detection Facts & Figures 2010. Atlanta: American Cancer Society, 2010.
2. McFarland EG, Levin B, Lieberman DA, Pickhardt PJ, Johnson CD, Glick SN et al. Revised Colorectal Cancer Screening Guidelines: joint effort of the American Cancer Society, U.S. Multisociety Task Force on Colorectal Cancer, and American College of Radiology. Radiology 2008; 248: 717-720.
3. Kentucky Cancer Registry. Cancer incidence and mortality rates by Appalachian Region in Kentucky, colon and rectum 2006. Data from the Kentucky Cancer Registry Inquire System. (Online) no date. Available: http://cancer-rates.info/ky/index.html (Accessed 5 August 2009).
4. Appalachian Regional Commission. Demographic and Health Information (Online) 2009. Available: http://www.arc.gov (Accessed 3 March 2010).
5. Davis RE, Armstrong DK, Redmond J, Dignan M, Norling GR. Evaluation of educational materials on colorectal cancer screening in Appalachian Kentucky. Preventing Chronic Disease 2006; 3(2): A43.
6. Kelly KM, Clarenda MP, Jenkins C, Norling G, White C, Jenkins T et al. Physician and staff perceptions of barriers to colorectal cancer screening in Appalachian Kentucky. Cancer Control 2007; 14(2): 167-175.
7. Centers for Disease Control and Prevention. Behavioral Risk Factor Surveillance System. Prevalence and trends data. (Online) 2009. Available: http://apps.nccd.cdc.gov/BRFSS/page.asp?cat=CC&yr=2002&state=KY#CC (Accessed 6 December 2010).
8. Bayliss E, Edwards A, Steiner J, Main D. Processes of care desired by elderly patients with multimorbidities. Family Practice 2008; 25(4): 287-293.
9. Grady KE, Lemkau JP, McVay JM, Carlson S, Lee N, Minchella M et al. Clinical decision-making and mammography referral. Preventive Medicine 1996; 25: 327-338.
10. Doescher M, Saver BG, Fiscella K, Franks P. Preventive care: Does continuity matter? Journal of General Internal Medicine 2004; 19(6): 632-637.
11. Heflin MT, Oddone EZ, Pieper CF, Burchett BM, Cohen HJ. The effect of comorbid illness on receipt of cancer screening by older people. Journal of American Geriatric Society 2002; 50(10): 1651-1658.
12. May DS, Kiefe CI, Funkhouser E, Fouad MN. Compliance with mammography guidelines: physician recommendation and patient adherence. Preventive Medicine 1999; 28(4): 386-394.
13. Klabunde CN, Vernon SW, Nadel MR, Breen N, Seeff LC, Brown ML. Barriers to colorectal cancer screening: a comparison of reports from primary care physicians and average-risk adults. Medical Care 2005; 43: 939-944.
14. Wee CC, McCarthy EP, Phillips RS. Factors associated with colon cancer screening: the role of patient factors and physician counseling. Preventive Medicine 2005; 41: 23-29.
15. Brenes GA, Paskett ED. Predictors of adoption for colorectal cancer screening. Preventive Medicine 2000; 31: 410-416.
16. Tashakkori A, Teddlie C. Mixed methodology: combining qualitative and quantitative approaches. Thousand Oaks, CA: Sage, 1998.
17. Appalachian Regional Commission. Data Reports 2010. (Online) no date. Available: http://www.arc.gov/data (Accessed 13 April 2010).
18. US Department of Health and Human Services: Health Resources and Services Administration. (Online) no date. Shortage Designation: HPSAs, MUAs & MUPs. Available: http://bhpr.hrsa.gov/shortage/ (Accessed 1 July 2009).
19. Dall T, Sen N, Zhang Y, Sahai N, Chen J. The impact of improved colorectal cancer screening rates on adequacy of future supply of gastroenterologists. Research Report Prepared for Olympus America Inc 2009. (Online) no date. Available: http://www.olympusamerica.com/CRCadvocacy/docs/Lewin-Gastroenterologist-Report.pdf (Accessed 15 April 2010).
20. Kentucky Institute of Medicine. Comprehensive Statewide Physician Workforce Study. Task Force Report 2007. (Online) 2007. Available: http://www.kyiom.org/pdf/KMAWorkforceReport9-24-07.pdf (Accessed 15 April 2010).
21. Pleis JR, Lucas JW, Ward BW. Summary health statistics for U.S. adults: National Health Interview Survey, 2008. Vital Health Statistics 2009; 10: 242. Available: http://www.cdc.gov/nchs/data/series/sr_10/sr10_242.pdf (Accessed 20 May 2010).
22. National Center for Health Statistics. Current estimates from the National Health Interview Survey: United States, Vol 3. Hyattsville, MD: National Center for Health Statistics, Department of Health and Human Services, 1986.
23. U.S. Preventive Services Task Force. Clinical guidelines: screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Annals of Internal Medicine 2008; 149(9): 627-637.
24. Smith RA, Cokkinides V, Brawley, OW. Cancer screening in the United States, 2009: a review of current American Cancer Society guidelines and issues in cancer screening. CA: A Cancer Journal for Clinicians 2009; 59: 27-41.
25. Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qualitative Health Research 2005; 15(9): 1277-1288.
26. Patton MQ. Qualitative research and evaluation methods. Thousand Oaks, CA: Sage, 2002.
27. Bernard HR. Research methods in anthropology. Walnut Creek, CA: AltaMira Press, 2002.
28. U.S. Census Bureau. State and county quick facts: Breathitt, Floyd, Knott, and Perry Counties, 2010. (Online) 2010. Available: http://quickfacts.census.gov/qfd/states/21000.html (Accessed 11 September 2010).
29. Smyth JM, Webb MS, Oikawa M. Self-Report of Cancer-Related Behaviors. (Online) no date. Available: http://cancercontrol.cancer.gov/brp/constructs/self-report/sr2.html (Accessed 26 June 2009).
30. Mirand AL, Beehler GP, Kuo CL, Mahoney MC. Physician perceptions of primary prevention: qualitative base for the conceptual shaping of a practice intervention tool. BMC Public Health 2002; 2: 16.
31. Centers for Disease Control and Prevention. Colorectal (Colon) Cancer: Screening Rates. Prevention and Early Detection: Keys to Reducing Deaths 2008. (Online) 2008. Available: http://www.cdc.gov/cancer/colorectal/statistics/screening_rates.htm (Accessed 5 August 2009).

