In 1994, the World Health Organization (WHO) incorporated Directed Observed Treatment (DOT) as a part of the Directed Observed Treatment Strategy (DOTS) in order to control tuberculosis (TB) globally1. Basic elements of DOTS included the prompt diagnosis of patients with active TB by sputum microscopy, a standard short-course combination chemotherapy of 6 months duration for newly diagnosed TB patients, a secure supply of drugs, and political commitment; however, the primary key was the direct observation of TB treatment (DOT) by a supervisor, usually a health or community worker2. Although the implementation of DOTS has been associated with a decrease in new TB cases globally, TB remains the most persistent and widespread infection worldwide, compounded by an upsurge in multidrug-resistant (MDR) TB cases3-5. Moreover, the consequences of interrupting the treatment contribute to drug resistance, prolonged morbidity and mortality6. In order to improve patients' adherence to treatment, DOT allows persons other than health staff to supervise TB patients and ensure they are taking their medication7,8.
DOT has been criticized as a passive model related to the success of health services in delivering only the appropriate drugs, but without taking into account the social component of the disease related to stigma, and not encouraging family members to modify living conditions and habits9. This comes as no surprise to researchers who have found no significant improvement to outcome between DOT and self-administered treatment10. Interventions, such as financial incentives11, reminder letters and increased supervision by staff may promote the adherence of patients to TB treatment, especially in drug users, minorities, and patients who are homeless12.
In Greece, TB is an obligatorily reported disease at local and regional level13. Pulmonary physicians are those mainly responsible for TB treatment. Every month, patients with TB must be taken from their homes to the pulmonary outpatient clinic, so physicians can evaluate their adherence to the treatment. The Hellenic Center for Disease Control and Prevention (KEELPNO) guidelines suggest medication administration for a period of 6 months, which has been applied in a great number of cases.
Data reported by KEELPNO have indicated a decline in the incidence of TB from 10.8 per 100 000 inhabitants in 1998 to 5.3 per 100 000 in 2002 for the whole of Greece, and from 8.8 per 100 000 to 1.6 per 100 000 for the specific region of Western Greece during the same period14. However, a recent study reported substantial underestimation of the TB burden in Western Greece15, indicating that there is an insufficient TB monitoring system in Greece. A study from Italy has reported similar results in the underestimation of incidence rates of TB16.
The purpose of this observational study was to evaluate the effectiveness of a modified DOT (MDOT) program conducted by a GP physician, as a pilot project in a rural region of Greece where there is substantial underreporting of TB cases15, in comparison to a self-administered approach towards TB treatment by residents of the same geographical area.
Study area and population
An observational study was performed in the Prefecture of Ilia, southwest Greece, in the period 2006-2009. The region covers an area of 2618 km2 and the population is estimated to be almost 200 000 inhabitants17.
Patients were recruited from the outpatient pulmonary clinic or the pulmonary ward of the main regional general hospital of Pirgos (GHP), where suspected TB cases were referred from other healthcare facilities (rural dispensaries, health centres) or private physicians practicing in the area.
The specimens obtained were transferred to the Department of Microbiology at the University Hospital of Patras (DMUHP).
All specimens received were processed in the DMUHP according to conventional microbiological procedures for identification of mycobacteria: after decontamination (BBL MycoPrep, Becton Dickinson, Cockeysville, Maryland, USA), cultures were performed by inoculation of sediments directly on Löwenstein-Jensen slants (L-J, bioMerieux, SA Lyon, France) and in BACTEC culture vials (Becton Dickinson). Identification at the species level was carried out by Polymerase Chain Reaction (PCR) and hybridization to commercial DNA probes (GenoType Mycobacteria, CM, Hain Lifescience, Nehren, Germany). All cases who tested positive by Ziehl-Nelsen stain were enrolled in the study.
Before sensitivity to TB medications had been evaluated, patients were treated with isoniazid (H), rifampicin (R), pyrazinamide and ethambutol for 2 months followed by a 4 month treatment with H and R18. Outcome assessment was performed by laboratory examination of sputum by the laboratory where the initial diagnosis was investigated. The outcome evaluation included six end-point measures: (i) cure, if sputum smears (3 samples) were negative; (ii) treatment completion at 6 months without smears results; (iii) treatment failure, if sputum smears remained positive or become positive again 2 or more months after treatment; (iv) death, if the patient died during the treatment, from any cause; (v) treatment interruption, if the patient did not comply with treatment for 2 consecutive months; and (vi) loss to follow up, if the patient transfered without notifying the GP or pulmonary physician18. Confirmed patients were referred from the pulmonary outpatient service to a GP physician, who implemented the MDOT program.
TB case finding, DOT program and treatment outcomes
MDOT was implemented in all newly detected cases during the period 2006-2009 by a GP from the nearest health center, supervised by a pulmonary physician at GHP. Previously-treated (since 2006) TB patients were identified from the GHP's records and enrolled as controls in the study. Home visits were conducted in both newly detected and previously treated cases.
During the first home-visit (within the first month of treatment), the GP informed the members of the family about the purpose of the visit, mainly focusing on issues regarding prevention, personal hygiene and living conditions. Then 0.1 ml Tuberculin PPDRT23SSI (Statens Serum Institute, Denmark; Mantoux test) was given to all household contacts who were not yet tested. The patient was trained to record on a calendar the date he received therapy and dates of required re-evaluation visits to the pulmonary outpatient clinic, and a family member was instructed to supervise and remind the patient of these actions. A questionnaire was completed by all household members regarding questions about sociodemographic characteristics, work and living conditions, all parameters with the potential to influence the development and outcome of the disease.
A second home visit was made to evaluate the Mantoux test results in family members who had been tested 3 days earlier. The presence of induration was determined by touch. The diameter of induration was measured transversely. The size of induration in mm greater than or equal to 10 mm was defined as true infection. Two weeks after the beginning of anti-TB therapy, the GP made a third visit to check medication compliance (monitoring the prescription booklet and the calendar records) and perform a physical examination in order to identify possible drug side-effects.
At the end of the second month, the GHP collected three samples of sputum in order to evaluate the success of treatment. Every 20 days within the treatment period the GP had visited the patients, both evaluating and supervising the treatment by counting the prescribed drugs. During the ninth and final home visit, a final evaluation based on clinical and laboratory test results was completed in conjunction with the attending pulmonologist at GHP, to assess the effectiveness of the therapy and the efficacy of the WHO-recommended strategy in comparison with self-administrated TB cases.
All diagnosed TB cases were reported to the regional public health department responsible for the collection of data regarding all notifiable diseases. Data from previous self-administered treatment patients (controls) included socioeconomic status, place of residence, and anti-TB treatment, collected from the GHP records. During the GP's home visit, data were collected concerning personal hygiene, size (m2) of the home, educational level, smoking and alcohol use habits. Additionally, the GP focused on collecting data regarding the completion of treatment received by patients in the past, and any preventive therapy given to relatives.
Pearson's χ2 test and Fisher's exact test were used to compare the groups, with the p-value set at <0.05.
Ethics approval
The study was approved by the plenary meeting of the Medical School of Patras and the Ethics Committee of the University Hospital of Patras. Written informed consent was obtained from all patients before enrolment.
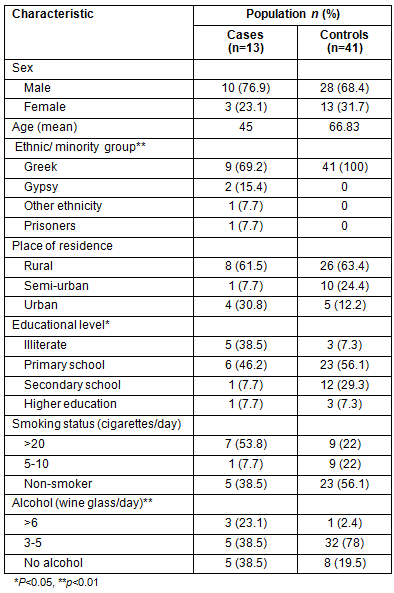
Demographic characteristics of the study population
Thirteen newly diagnosed TB patients (cases) and 41 past-treated TB cases (controls) were included in the study. Thirty close contacts of the newly diagnosed TB patients and 111 close contacts of past-treated TB subjects were also included. Each case was matched to three controls according to age and sex. Significant statistical differences between the two groups were observed regarding ethnic/minority group (p=0.002) and educational level (p=0.039), with higher rates found among those who were illiterate and those who were Gypsies (Table 1). In the study, 69.2% of the cases and 87.8% of controls were permanent residents in rural and semi-urban areas, and most were illiterate, as determined by their level of education. In addition, 5 of 13 TB cases belonged to minority groups.
Table 1: Demographic characteristics of the study population
(newly-diagnosed [cases] and previously-treated [controls] TB patients)
Microbiological results
Eleven of 13 patients tested positive for TB (Mycobacterium tuberculosis [ΜΤΒ], PCR test) in the clinical specimens, one additional patient had a negative PCR result while another was not tested molecularly. Nine of 13 MTB isolates were sensitive to the agents tested by both methods, one isolate was streptomycin-resistant, one ethambutol-resistant, one isoniazid-resistant and another was resistant to isoniazid and streptomycin. All 30 household contacts proved negative for MTB by molecular and phenotypic methods.
Treatment outcome
The treatment outcome of 13 cases who entered the MDOT program during the study period was analyzed. The treatment success rate was 11 (84.6%) cases (cured or treatment completed), one case (7.7%) died during treatment, one case (7.7%) was lost during follow up, and none interrupted treatment (Table 2).
Thirty close contacts were given a Mantoux test. Before the implementation of the MDOT program, the Mantoux test was carried out by the chest physician on 17 close contacts of TB patients, whereas the GP identified 13 more close contacts on the first visit of the MDOT program. In the control group, the Mantoux test had not been applied in 59 of 111 close contacts (Table 3).
In four (16.6%) close contacts of TB patients, chemoprophylaxis was given by the GP. In comparison, 14 (12.6%) close contacts of the control group received chemoprophylaxis and seven (6.3%) a complete anti-TB regimen because of active TB (Table 3).
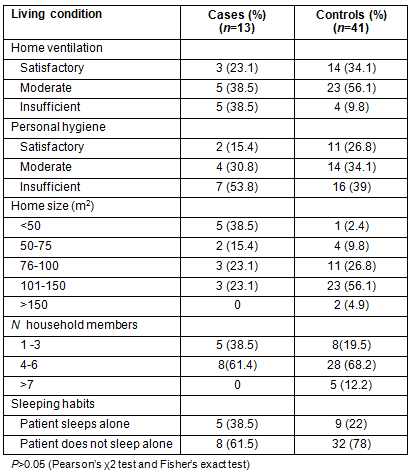
The living conditions of patients with TB which can influence the disease development and outcome assessment, are presented (Table 4).
Table 2: Treatment outcomes and effectiveness of modified
directly observed treatment program in newly-diagnosed
cases during the study period, 2006-2009 (N=13)
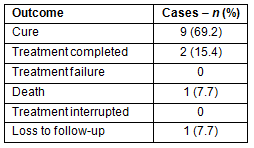
Table 3: Characteristics of household contacts of newly-diagnosed
(cases) and previously-treated (controls) TB patients
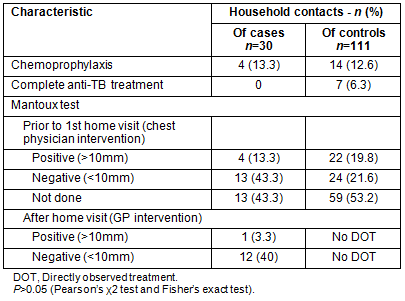
Table 4: Living conditions in cases and controls
Discussion
The main findings in our study were a cure rate of 69.2%, and that the successful outcome (treatment completion with or without microbiological confirmation) in patients under DOT was 84.6%.
The main intention of the present study was not only to apply more flexible DOT through a GP at home, and not restricted to supervising the treatment regime, but also to identify persons who are at risk of developing active disease and to modify conditions that affect TB vulnerability.
In this context, the analysis of results showed that a trained GP is able to follow up patients with TB and identify factors affecting transmission, such as personal and home hygiene, and reduce infectivity during close contact with TB patients. As the analysis of our study showed, none of the close TB contacts were affected by TB. In contrast, seven control close contacts reported active TB disease.
In Europe, according to the WHO report of 2006, the majority of countries reported a decline or stabilization in TB notification rates19, but when focusing on individual countries there are disturbing results which may impact TB incidence in the future. In Ireland, Italy and Norway, there has been an increase in infection rates in young adults. In Sweden, an increase of TB incidence, especially among asylum seekers and refugees, was noted; while in the UK, foreign-born individuals have mainly been responsible for the increase in TB notification rates since 200519.
Regarding the efficacy of treatment regimen, the working group of 'WHO against TB' and the 'International Union against Tuberculosis and Lung Disease'19 issued the statement that treatment monitoring is essential for the evaluation of treatment results and must be incorporated in national TB control programs. Moreover, factors that increase the incidence of TB and multidrug-resistant TB (MDR-TB) include individual biological factors (genetic factors or TB interaction with other infectious diseases), social status and living conditions (poverty, crowding, poor nutrition), environmental factors (migration, homelessness, asylum seekers, alcohol use) and the ability of a healthcare worker in any health system to ensure a prompt diagnosis of TB20-24. In this context, MDR-TB affects treatment outcomes in Europe25. Other studies have pointed out that MDR-TB could threaten the control of TB globally, and emphasize the role of a quality DOT program in reducing TB incidence globally26,27.
In the present study, 5 of 13 cases belonged to minority groups, so the close supervision of treatment success may be of great importance. It is well known that persons who belong to minorities and move from their place of residence are more likely to interrupt TB treatment. Studies from Western countries have reported that TB treatment failure was greater among those who were homeless, alcoholics, HIV-infected patients and injecting drug users28,29.
Another important factor which can affect the adherence of patients to TB treatment is the residence of TB patients. Although this factor is well known for the low income countries8, in Western countries it is not well understood. Recently, Alvarez et al reported socioeconomic inequalities in TB mortality by level of education in urban and rural populations in several European countries30. In spite of the significant overall treatment success of the DOTS strategy, inconsistent results in case detection have been reported31. According to Bishai et al, the upsurge of MDR-TB could be related to high detection rates of TB coupled with non-compliant patients or low case detection rates associated with high-compliance patients5.
The present results indicate that the implementation of a MDOT by a GP in Greece can combine treatment success with case detection. Moreover, a more flexible DOT program implemented by a GP may be incorporated into the National Tuberculosis Program, especially in rural areas and social communities which run the risk of developing TB.
Limitations
The main limitations of the present study were the recall bias of previously treated patients, lack of information about HIV infection and the small sample of TB cases. Another bias may be that 30% of the newly diagnosed TB cases belonged to minority groups, whereas all identified previously-treated cases were native Greeks. This may be because many native Greeks with TB prefer to visit hospitals outside their residential region to avoid being socially stigmatized.
The total number of TB cases reported to KEELPNO that were diagnosed either at PGH at other healthcare facilities in Greece, but were residents of the Prefecture of Ilia, amounted to 45 cases for the period 2006 to 2009. Of these, 13 (29%) cases were diagnosed at PGH, therefore only these recruited patients had been enrolled in the pilot DOT study. Thus, it cannot be ruled out that the high treatment success in the present study may be overestimated. Furthermore, the very small sample size does not allow for generalization. Hence, suggesting this as a possible treatment model is not appropriate. Indeed, the study was conducted in a country with a comparatively low incidence of TB where DOT is routinely implemented. However, since TB is increasing in Greece, mainly due to the high level of immigrants from countries with a high incidence of TB, it is conceivable that DOTS could become a treatment model in the future. In order to obtain more reliable results, the model needs to be implemented in more rural regions of Greece and this needs political commitment.
Finally, it was not possible to estimate the cost of implementing this MDOT program through a GP. However, other studies have provided evidence that implementation of DOTS by community health workers achieves high cure rates with a lower cost per case than conventional DOTS implementation in health centers32,33. Notwithstanding the fact that a GP could be replaced by a skilled nurse, thus possibly reducing the cost, in Greece the health system is not organized for home nursing by specialized staff other than physicians.
Conclusion
In this pilot program, a GP was able to improve patient compliance with TB control strategies in order to control TB transmission. Further studies with appropriately skilled health staff in the European Union, especially in rural areas, are needed to establish the effectiveness of this MDOT program in high-income countries.
References
1. WHO. Framework for effective tuberculosis control. WHO/TB/94.179. Geneva: World Health Organization, 1994.
2. Anuwatnonthakate A, Limsomboon P, Nateniyom S, Wattanaamornkiat W, Komsakorn S, Moolpate S et al. Directly observed therapy and improved tuberculosis treatment outcomes in Thailand. PLoS One 2008; 3(8): 30-89.
3. Raviglione MC. The TB epidemic from 1992 to 2002. Tuberculosis 2003; 83(1-3): 4-14.
4. Chakroborty A. Drug-resistant tuberculosis: an insurmountable epidemic? Inflammopharmacology 2011; 19(3): 131-137.
5. Bishai JD, Bishai WR, Bishai DM. Heightened vulnerability to MDR-TB epidemics after controlling drug-susceptible TB. PLoS One 2010; 5(9): e12843.
6. Cuneo WD, Snider DE. Enhancing patient to compliance with tuberculosis therapy. Clinical Chest Medicine 1989; 10: 375-380.
7. Clarke M, Dick J, Zwarenstein M, Lombard CJ, Diwan VK. Lay health worker intervention with choice of DOT superior to standard TB care for farm dwellers in South Africa: a cluster randomised control trial. International Journal of Tuberculosis and Lung Disease 2005; 9(6): 673-679.
8. Egwaga S, Mkopi A, Range N, Haag-Arbenz V, Baraka A, Grewal P et al. Patient-centred tuberculosis treatment delivery under programmatic conditions in Tanzania: a cohort study. BMC Medicine 2009; 21(7): 80.
9. Smith AJE, Moore LM. Selective directly observed therapy: Appraisement and perception of likely participants. Journal of Public Health Management Practice 1995; 1: 14-21.
10. Volmink J, Garner P. Directly observed therapy for treating tuberculosis. Cochrane Database of Systematic Reviews 2007; 17(4): CD003343. DOI: 10.1002/14651858.CD003343.pub3.
11. Volmink J, Matchaba P, Garner P. Directly observed therapy and treatment adherence. Lancet 2000; 355: 1345-1350.
12. WHO. Adherence to long term therapies: evidence for action. Geneva; World Health Organization. (Online) 2003. Available: http://www.who.int/chp/knowledge/publications/adherence_full_report.pdf (Accessed 8 March 2013).
13. Hellenic Center for Disease Control & Prevention. Diseases/ Health Topics Flu, HIV/AIDS, Hepatitis, Cancer, Tuberculosis. (Online) no date. Available: http: //www.keelpno.gr (in Greek).
14. Hellenic Center for Disease Control & Prevention. Epidemiological / Statistical Data. Data for diseases, weekly - annual reports. Tuberculosis, epidemiological data for tuberculosis in Greece. (Online) no date. Available: http: //www.keelpno.gr (in Greek).
15. Jelastopulu E, Alexopoulos EC, Venieri D, Tsiros G, Komninou G, Constantinidis TC et al. Substantial underreporting of tuberculosis in West Greece: implications for local and national surveillance. Eurosurveillance 2009; 14(11): 14-17.
16. Baussano I, Bugiani M, Gregori D, van Hest R, Borraccino A, Raso R et al. Undetected burden of tuberculosis in a low-prevalence area. International Journal of Tuberculosis and Lung Disease 2006; 10(4): 415-421.
17. Hellenic Statistical Authority (EL.STAT). Statistical database (Online 2011). Available: http: //www.statistics.gr/portal/page/portal/ESYE/PAGE-database (Accessed 1 May 2011).
18. Migliori GB, Raviglione MC, Schaberg T, Davies PD, Zellweger JP, Grzemska M et al. Tuberculosis management in Europe. Recommendations of a Task Force of the European Respiratory Society (ERS), the World Health Organisation (WHO) and the International Union against Tuberculosis and Lung Disease (IUATLD) Europe Region. European Respiratory Journal 1999; 14: 978-992.
19. EuroTB and the National Coordinators for Tuberculosis Surveillance in the WHO European Region. Surveillance of tuberculosis in Europe. Report on tuberculosis cases notified in 2006. Saint-Maurice, France: Institut de Veille Sanitaire, 2008.
20. Dye C, Watt CJ, Bleed DM, Hosseini SM, Raviglione MC. Evolution of tuberculosis control and prospects for reducing tuberculosis incidence, prevalence, and deaths globally. JAMA 2005; 293: 2767-2775.
21. Centis R, Ianni A, Migliori GB. Evaluation of tuberculosis treatment results in Italy, report 1998. Monaldi Archives for Chest Disease 2000; 55: 293-298.
22. Helbling P, Medinger C, Altpeter E, Raeber PA, Beeli D, Zellweger JP. Outcome of treatment of pulmonary tuberculosis in Switzerland in 1996. Swiss Medical Weekly 2002; 132: 517-522.
23. Diel R, Neimann S. Outcome of tuberculosis treatment in Hamburg: a survey, 2001. International Journal of Tuberculosis and Lung Disease 2003; 7: 124-131.
24. Dye C, Watt CJ, Bleed DM, Williams BG. What is the limit to case detection under the DOTS strategy for tuberculosis control? Tuberculosis 2003; 83(1): 35-43.
25. Faustini A, Hall AJ, Perucci CA. Tuberculosis treatment outcomes in Europe: a systematic review. European Respiratory Journal 2005; 26: 503-510.
26. Pablos-Mendez A, Gowda DK, Frieden TR. Controlling multidrug-resistant tuberculosis and access to expensive drugs: a rational framework. Bulletin of the World Health Organization 2002; 80: 489-500.
27. Dye C, Williams BG, Espinal MA, Raviglione MC. Erasing the world's slow stain: strategies to beat multidrug-resistant tuberculosis. Science 2002; 295: 2042-2046.
28. Pablos-Mendez A, Knirsch CA, Barr RG, Lerner BH, Frieden TR. Non-adherence in tuberculosis treatment: predictors and consquences in New York City. American Journal of Medicine 1997; 102(2): 164-170.
29. Cummings KC, Mohle-Boetani J, Royce SE, Chin DP. Movement of tuberculosis patients and the failure to complete antituberculosis treatment. American Journal of Respiratory and Critical Care Medicine 1998; 157: 1249-1252.
30. Alvarez JL, Kunst AE, Leinsalu M, Bopp M, Strand BH, Menvielle G et al. Educational inequalities in tuberculosis mortality in sixteen European populations. International Journal of Tuberculosis and Lung Disease. 2011; 15(11): 1461-1467.
31. Obermeyer Ζ, Abbott-Klafter J, Murray CJL. Has the DOTS strategy improved case finding or treatment success? An empirical assessment. PLoS One 2008; 3(3): 17-21.
32. Khan MA, Walley JD, Witter SN, Imran A, Safdar N. Costs and cost-effectiveness of different DOT strategies for the treatment oftuberculosis in Pakistan. Directly observed treatment. Health Policy Planning 2002; 17(2): 178-186.
33. Palacios E, Guerra D, Llaro K, Chalco K, Sapag R, Furin J. The role of the nurse in the community-based treatment of multidrug-resistant tuberculosis (MDR-TB). International Journal of Tuberculosis and Lung Disease 2003; 7(4): 343-346.
