Invasive cervical cancer (ICC) remains an international concern, although the prevalence and mortality of ICC has decreased considerably in most Western nations, with some exceptions. One of these exception is Appalachian Kentucky, where ICC incidence and mortality rates are approximately 67% and 33% higher than US averages, respectively1,2. Almost 25% of eligible Appalachian women, compared with 16.6% nationally, have not had a Pap test consistent with US Preventive Services Taskforce guidelines1. These guidelines recommend that women aged 21 to 65 years receive Pap tests every 3 years or, for women aged 30 to 65 years who want to lengthen the screening interval, receive screening with a combination of cytology and human papillomavirus (HPV) testing every 5 years3. Breast cancer, a continued concern among Western and non-Western nations alike, also disproportionately affects Appalachian women4. Although breast cancer incidence rates are lower among Appalachian women compared with the national average, mortality rates are higher (24/100 000 in the US vs 25.2 in Appalachian Kentucky), suggesting inadequate screening, leading to a later stage of diagnosis and perhaps suboptimal treatment5. Approximately 40% of Appalachian women aged 50 years and older have not had a mammogram or clinical breast examination during the past 2 years compared with a national average of 29%6. In the USA, biennial breast cancer screening with mammography is recommended for women aged 50-747.
Appalachian women's explanations for inadequate screening tend to be consistent with three main categories of influences on cancer screening (personal, professional, and systemic)8. Personal factors include fear of the procedures and the screening outcomes, privacy concerns, and embarrassment9,10. For example, the authors' previous work indicated that a meaningful barrier to Pap tests in small, tight knit rural communities involves women's reluctance to subject themselves to an uncomfortable and embarrassing medical procedure conducted by their former high school classmates11. Katz et al corroborated this point, noting that Appalachian residents often travel to neighboring counties rather than undergo these examinations by those familiar to them10. Similarly, women who are obese or who engage in unhealthy behaviors such as smoking report wanting to avoid medical scrutiny12.
Insufficient personal resources and stressful life events comprise additional barriers to screening. Women report feeling unable to obtain screening if they lack health insurance and access to adequate transportation12-14. As has been previously documented, competing demands for time and monetary resources, particularly in the absence of symptom and provider recommendation, impedes women from obtaining cancer screening15. Additionally, in their examination of over 500 Appalachian Ohio women, Paskett et al found that those who had experienced major life events were significantly less likely to obtain Pap tests according to guidelines16. Professional and systemic barriers include health professional shortages, insufficient quality and continuity of care, and lack of physician recommendation, sometimes due to the provider's perception that screening is not feasible because of patients' lack of insurance, low socioeconomic status, perceived cancer fatalism, or disinterest in prevention17.
Such explanations for inadequate cancer screening behavior lend themselves to interpretation based on consumer information processing (CIP) theory18. Consumers of healthcare services have limited information processing capacity, will downplay negative information (eg fears about screening and privacy) and follow their preconceived notions (eg that one must have many resources to obtain screening). Women are therefore less motivated to search for information regarding breast and cervical cancer, screening, or means of overcoming personal, professional, or systemic barriers to screening. However, CIP theory indicates that when health information is available and easy to processes, patients can be guided to engage in better health behavior19. This article builds on the authors' previous work and that of others, that has broadly identified personal, professional, and systemic influences on women's cancer screening to update and capture, from a range of perspectives (patient, social service providers, healthcare professionals etc), specific challenges to obtaining screening. The goal of this study was to inform the development of appropriate interventions capable of addressing the grounded and current insights of Appalachian residents. Evidence-based interventions to promote cancer screening range from client reminder systems, innovative approaches to decreasing out-of-pocket expenditures, and one-on-one educational approaches20.
Setting
In 1965, the United States Congress enacted legislation that designated a large geographic expanse from New York to Mississippi as 'Appalachia'. The Appalachian region includes 13 states, 420 counties, 23 million residents, and approximately one out of 12 Americans. Forty-two percent of Appalachia's residents are rural, compared with 20% of the rest of the nation21. Appalachian Kentucky is located in the center of the region and, along with West Virginia and Tennessee, brings to mind many descriptions of Appalachia: beautiful scenery, strong family connections, and resource deficiencies. Along with numerous assets, the residents of these counties maintain health and socioeconomic status indicators significantly lower than the national averages21.
Sample recruitment
To obtain insights to inform the development of such interventions, focus groups (FG) and key informant (KI) interviews were conducted with diverse community members. Given the high proportion of active church participation in the region (especially among women)22, most of participants in the FG came from churches. Local community staff spoke with church leaders from diverse congregations in the study location (a five-county region in eastern Kentucky), arranged a convenient meeting time, and informed congregants about the opportunity to participate in an FG.
Several African-American churches were targeted in order to ensure representation of African-American women, a group that tends to be doubly disadvantaged. This recruitment effort resulted in the sample consisting of 8% African-American participants, more than twice this population's presence in the counties23. Inclusion criteria included being female, aged 18 years and older, and willing and able to participate in an FG. Given US Preventive Services Taskforce Pap test recommendations at the time, 18-20 years-olds were included24; however, updated guidelines released in 2012 recommend initiation of Pap tests at age 213. The sample size was guided by principles of theoretical saturation25, and a total of 6 FG were conducted before reaching saturation. Since two additional FG were already scheduled, these sessions served as an opportunity to confirm findings.
Two community staff members conducted 19 KI interviews to obtain insights from local stakeholders who, through their professions and affiliations, could offer insights on cervical and breast cancer screening determinants in rural Appalachia and discuss programming to increase screening. Key informants were selected through snowball sampling26. Inclusion criteria entailed being 18 years and older, willing and able to participate in an interview, and having appropriate medical, social service, or regional expertise.
Protocols
Between July 2009 and January 2010, two trained community staff moderated and two other staff members co-moderated the FG and KI sessions. The moderators began the sessions by describing the purpose of the sessions, explaining and then collecting informed consent, and asking open-ended questions developed by academic researchers and community members. Given time constraints, inquiries were not made about screening history, awareness of community programs to facilitate screening, or other salient issues. A sample segment of the discussion guide is provided (Fig1). Participants completed sociodemographic forms and paperwork for a US$25 honorarium. Most sessions were of 90-120 min duration.
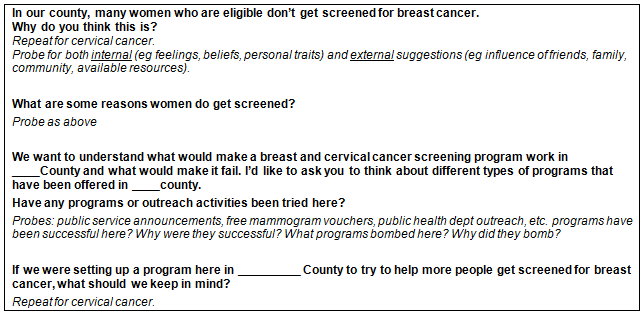
Figure 1: Example of focus group interview guide.
Analysis
To ensure rigor and project fidelity, the same trained moderators and interviewers conducted the FG and KI interviews; member checking was employed on completion of each session; and a codebook was developed and used in order to standardize data analysis27. With prior approval from participants, all sessions were tape recorded, transcribed by local, trained transcriptionists and reviewed for accuracy by the community staff. NVivo software (www.qsrinternational.com) was used for coding, organization, and analysis. The analysis process began with two trained qualitative researchers independently engaging in line-by-line coding of the transcripts, with one affixing codes to each text segment then the other refining and defining the codes. The two researchers each developed a separate, preliminary codebook and merged the codebook, improving coding standardization and recording definitions and operationalization of codes. The researchers refined the codebook six times.
Ethics approval
The University of Kentucky Institutional Review Board approved all human subjects protection protocols (08-119-P2H).
Sample characteristics
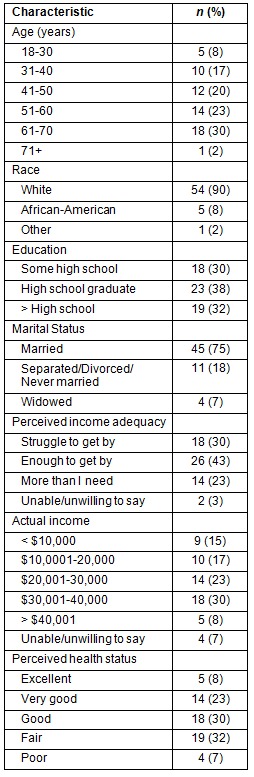
The FG sample (Table 1), was similar to demographic profiles of Appalachian residents; the majority were White (90%), of lower socioeconomic status, married (75%), and perceived their health to be very good (23%), good (30%), or fair (32%). Thirty percent of the sample indicated they were financially struggling to get by, 43% felt they had enough to get by, and 23% felt they had more than they needed. A majority (55%) reported annual household incomes below $30,000, and two-thirds had achieved high school education or less. Participants from FG ranged from 18 to 71 years.
Table 1: Focus group participants' characteristics (N=60)
Most KI were female, with ages ranging from 25 to 64 years. Three KI were physicians who perform cancer screening, four key informants worked as Cooperative Extension agents, seven were local health department employees, two were cancer survivors, two were ministers, and one was involved in a cancer coalition. Given their professional status, most KI had educational and income levels above average for the region.
Themes
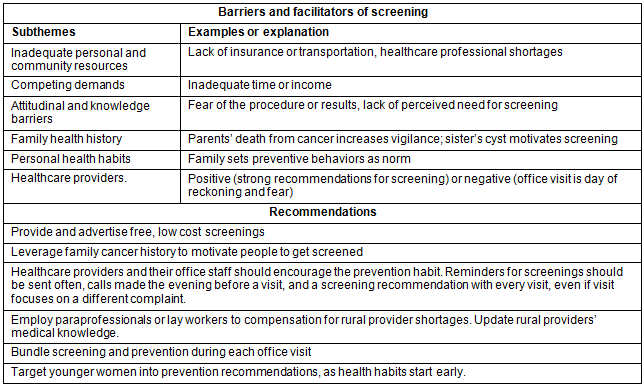
Both KI and FG participants described barriers to and facilitators of screening that were consistent with the existing literature. These included inadequate personal and community resources (eg lack of insurance or transportation, healthcare professional shortages) and competing demands (eg inadequate time or income); and attitudinal and knowledge barriers (eg fear of the procedure or results, lack of perceived need for screening). Results also revealed less commonly articulated themes in the Appalachian cancer screening literature, including how cancer screening can be influenced by family health history, personal health habits, and healthcare providers. Research participants articulated recommendations for increasing female cancer screening in the region (Table 2).
Table 2: Summary of themes, subthemes, and examples from focus groups and key informant interviews
Personal and community resources: Participants expressed a lack of insurance and financial resources as barriers to breast and cervical cancer screening. In this high-poverty region of Central Appalachia, a KI suggested that 'If it was free more people would be willing to go'. Another woman offered:
My mother always went because of the $50 [cancer screening] program at the hospital, otherwise it would cost way more because her insurance didn't cover it.
Another suggested:
I don't know if there is the funding for offering free exams for people who would qualify once a year but a lot of people with this economy are out of work or don't have good pay or full-time work, so I believe people would take advantage of a program that had a special rate.
However, one FG participant suggested that even low-cost screenings are not enough:
I think its affordability too. It's hard to get screened because, yeah it may only be $40 for a mammogram but then what if they find something and you still don't have insurance? You have to think of the long run.
Other participants suggested these low-cost services are available if one knows where to look. One KI said, 'There are really good resources out there, but people just don't know about them.' An FG participant supported this statement, indicating:
I think a lot of people can't afford to go to the clinic, yet a lot of the fliers and advertising are at the clinic. So if people can't afford to go to the clinic they won't even see those fliers.
Even when screening services are available at free or low-cost, transportation and free time often serve as a barrier to screening. One KI indicated:
They may not have access to get there, whether its transportation-wise or they have children or work obligations they may not be able to find the time and work something out so they can go.
One participant recalled a free program that drives women directly to the screening facility, but said that this program is not well advertised:
For instance, the extension office has a cervical cancer screening program in which they take ladies to Lexington once a month. They take a van load of women and it's all free. But how many women actually know about that?
Even when personal and community resources are available, attitude and knowledge still play a pivotal role breast and cervical cancer screening in this community.
Attitudes and knowledge: Participant's attitudes and knowledge served as both barriers to and facilitators of cancer screening. For example, KI and FG participants alike believed that community members do not care about cancer screenings, stating:
I don't know if it's just a nuisance to them...they just feel like that's the last thing on their mind, worrying about whether they get a Pap or a mam [mammogram].
One FG participant indicated, 'If they have one once and it comes back negative they feel it's a waste of time to go through it again'. Sometimes, the attitude of family members serves as a screening barrier, as reported by one FG participant: 'I know a woman whose husband wouldn't let her go, and she died with it'.
Women also described their community members as having a lack of cancer screening knowledge; however, they rarely referred to themselves as being unknowledgeable. Instead, as a women reported, '...young women have a tendency to think that this is not going to happen to me. And it does'. One FG participant tied lack of screening to a widespread avoidance of physicians and clinics in the region, stating:
It is pretty scary most especially if you do not have insurance. Most of us don't even go to the doctor until we have a problem. If it ain't hurting or bothering us then we just don't go. We could have a lot of things that starting out do not hurt until it is too late.
However, attitude and knowledge were also strengths among some community members. One KI reported:
I have always been faithful to mine because from one year to the next things can really change drastically and that is why I feel like you should get screened every year.
Family history of cancer: Knowledge of family history was also a facilitator to breast and cervical cancer screenings. Participants described how family members' cancer diagnosis and treatment increased their sense of vulnerability and caused them to prioritize obtaining screenings. An FG participant described her vigilance in receiving annual breast and cervical cancer screenings noting, 'My mom and dad both died from lung cancer so we always have cancer awareness in the back of our minds'. However, one KI noted that her family's health history made her complacent about her own breast health:
My older sisters have all had small cysts so when I found mine, that is what I thought it was. I wasn't worried; I just thought to myself, 'I need to watch it.' Turns out, the lump was cancerous.
Similarly, a healthcare provider speculated as to why her colleagues failed to encourage her older sister to initiate mammograms, saying:
I think she was let go for so long because of her age [69] and also because there are no cases of female cancer in our family.
Personal health habits: Many participants described how obtaining a Pap test or mammogram was strongly influenced by personal habits, many of which stemmed from family behaviors, as described by one FG participant:
I think a big thing is parents. If you are a young girl, and your mom encourages you [to get a Pap test], that's something you make sure to do every year.
Habits also seemed to derive from perceived threat, as another FG participant observed:
I have always been faithful to mine [annual screening], because from one year to the next, things can really change drastically...
Conversely, lack of routine and habitual screening was a barrier. As one FG participant noted:
Before I ever had a Pap test, I was afraid because it was something I never experienced even though it was so important. It's hard to get someone to go if they have never been.
Healthcare providers: In both positive and negative ways, healthcare providers exerted a substantial influence on screening behavior. Many participants described how their provider's recommendation was the key motivator for them to get screened, as in the case of one FG participant: 'If my doctor had not been so insistent on me getting one [mammogram], I would not have done it'. After not receiving a Pap test for many years, an FG participant's positive experience with a nurse practitioner encouraged her to get regular screenings:
It's not as scary as everyone thinks it is. I went with my friend when she was having one, and it lasted maybe five minutes. Then I decided to have one after she talked to me, and having the nurse there, too, helped. She was patient and let me feel the equipment and told me what she was doing and went through every step.
However, for the many women who lacked regular screenings, the doctor's office visit was a day of reckoning, sometimes fraught with fear or confusion:
Once the doctors get you in there, they sometimes have a way of making you think you are going to die, and once you are let go, you never want to go back.
The challenge of actually getting to see a healthcare provider and having him or her speak in 'normal language' led one FG participant to suggest:
I think it would help people a whole lot if they just had a way to answer all of their cancer screening questions without having to go to the doctor!
Recommendations: Participants made several recommendations, all relevant to providers, pertaining to these key influences on breast and cervical cancer screening. Providing, and heavily advertising, free or low-cost screenings was considered essential for increasing female cancer screening rates in the community. Leveraging family cancer history was also viewed as a useful strategy for healthcare providers and patients alike. Many participants suggested that healthcare providers should educate patients on their potential susceptibility to cancer because of family history:
If my doctor asked me about my relations, he would know that everyone's had cancer just about. If I needed any motivation, all he would have to say is, 'D, you are at high risk of cancer. You need to get tested every year.' And I'd sit up and listen.
Another set of recommendations pertained to the need to develop a habitual approach to prevention. Although the family was viewed as the origin of such habits, healthcare providers were seen as proximal influences, steering patients into screening. Many participants suggested that healthcare providers should start early in an effort to educate and engrain screening habits and establish screening as a normative activity. Furthermore, to enhance efficiency and reduce patient anxiety, some participants encouraged a one-stop shopping approach or 'bundling' of cancer screening. As an FG participant noted:
I went for other things but she [the physician] talked me into the mammogram because it was free, and I got a Pap smear then, too.
Participants made several recommendations on how to supplement the considerable healthcare provider influence on screening. One FG participant suggested that a paraprofessional (such as an licensed practical nurse, a certified educator or even a receptionist) could initiate the conversation regarding cancer screening, so that when the patient saw the doctor:
...the pump would be primed and she [the patient] could just talk it over. That would save time and let the doctor know that she is interested in getting screened.
Others emphasized the need for receptionists to send reminders, call the day before to remind about appointments, and for healthcare providers to be persistent about recommending screening, even when patients are visiting for other complaints. One FG participant described how screening was recommended to her:
I was there for another reason, and they just happened to ask me when my last Pap was, and I told them that I was due one. So that is when she [the physician] gave me an appointment to have that done.
Many participants also suggested that healthcare providers include younger women when providing information and recommendations for cervical and breast cancer screening, rather than the women aged 40-64 years who are most often targeted. However, negative perceptions of healthcare providers led some participants to suggest substituting a lay health advisor for certain health promotion activities. Regarding educational counseling, a key informant recommended involving lay health advisors, noting:
I think it's a good idea because some people have these doctor phobias. If they are more informed in their comfortable surroundings, they would be more likely to have the screenings done.
Additionally, many FG and KI participants were skeptical about how up-to-date some rural healthcare providers might be, suggesting that '...sometimes a little refresher might be good'.
Discussion
A novel concept to emerge in this study was the role of providers in educating and encouraging screening uptake in Appalachia, although existing literature corroborates participants' perspectives that providers are extremely influential in cancer screening uptake in other regions of the world. In diverse populations that also experience challenges obtaining screening, provider recommendation remains a major facilitator of screening28. Such influence includes the power and acceptability of a recommendation8, which are key components of effective interventions based on CIP theory19. The effectiveness of cancer education overall is highly dependent on the acceptability of the educator and provider. As participants noted, culturally appropriate and comprehensible messaging was extremely important to their screening decision-making. As CIP theory predicts, environmental cues (such as culturally relevant and intelligible messages put forth by acceptable providers) serve to enhance consumer motivation to gather and attend to information about cancer screening18. Unlike the present authors' previous work on colorectal cancer screening14, most participants in the present study were aware of the need for cervical and breast cancer screening, but suggested that additional motivation and encouragement from providers would improve the likelihood of screening. Consistent with this suggestion, client reminder systems have been shown to be effective in increasing mammograms and Pap tests in diverse populations20. For example, in one trial, women eligible to receive a mammogram from a primary-care practice network received either personalized letters or emails to remind them to schedule a mammography appointment, an informational brochure, and a follow-up phone call if they did not respond to reminders within a specified time (treatment group), while those in the usual care group received none of these reminders. Sixty-four percent of those in the treatment group (vs 55.3% in the usual care group) received a mammogram (p<.001)29.
In addition, providers play a key role as conduits for encouraging the collection and use of family history as a risk-stratification tool. Acquiring and evaluating one's family history with the assistance of a qualified provider can help patients gather sufficient data about their cancer risk and screening options, without making premature and erroneous decisions (eg there is nothing to be done) prior to gathering sufficient information. Participants in this study supported previous findings that discussion of family history may be a valuable tool for encouraging screening30. Currently, few providers in the USA discuss their patients' family history with them, and still fewer document such history in patient records31. Given that collecting family history is inexpensive, can be undertaken by paraprofessionals or by computers in office kiosks, is considered acceptable by patients, encourages consideration of shared genetic and environmental factors, and theoretically supports appropriate consumption of screening opportunities, such an approach may be readily implemented19. An increasing evidence base corroborates this approach; one of two groups in a randomized trial of 901 women with at least one first-degree relative with breast cancer were mailed materials identifying their personal risk of breast cancer. The intervention groups had an 8% greater likelihood of obtaining a mammogram than those in the control group32.
Participants also emphasized the key role that habits play in screening adherence, and existing literature corroborates this point. For example, those who obtain annual exams are more likely to receive cancer screenings as well as other types of preventive services33, indicating that they learned from one experience (an initial preventive service), which affected future screening behavior, as CIP theory would predict18. Change of existing suboptimal habits or establishment of beneficial habits may need to be initiated at key 'teachable moments' when patients are likely to be amenable to processing information about consumption/adoption of risk-reducing behaviors. For example, McBride et al determined that smoking cessation was more likely to occur during pregnancy, hospitalization, and disease diagnosis than for routine office visits and even abnormal test results34. Pertaining to cancer screening specifically, Bellizi et al found that cancer survivorship itself constitutes a 'teachable moment', with cancer survivors being one-third more likely than controls to meet mammogram and Pap screening recommendations35. Other teachable moments that may instill lifelong habits include cancer diagnosis of family members and routine office visits to care for multiple chronic conditions36. Patients are more likely to recall recommendations given during routine office visits than those received via the media, and when receiving a health behavior-related diagnosis, recall improved two-fold37. As suggested by the participants in this study, implementation of creative office-based programming, like 'The Cancer SOS' intervention38 or opportunistic screening efforts, holds particular promise for underserved populations.
Participants suggested that bundling preventive services represents another promising provider-related approach that is again supported by CIP theory and has been suggested by previous (but distinct) participants from the authors' Appalachian research efforts14. Bundling refers to the concept of packaging services together, that is, offering various cancer screenings within a single health visit39, which would reduce consumers' (patients') information processing load as they would not have to engage in multiple searches to acquire information regarding various screening options. Women who go to their healthcare provider for preventive health maintenance visits are more likely to receive screening for breast cancer, suggesting that prevention behaviors can be bundled to increase screening rates40. Similarly, regular clinical breast exams and Pap smears tend to be the best predictors of receipt of mammography41. Bundling may also reduce potential transportation and financial barriers to screening, decrease co-payments, and reduce lost wages. Moreover, bundling may make the receipt of multiple screenings seem more feasible, particularly for those rural residents who have to travel greater distances to receive their care. Bundling does, however, come with some risk; it is possible that too many services in a single visit may be overwhelming for a patient, and if patients are dissatisfied with their visit, they may be less likely to engage in any type of cancer screening in the future39. Currently, there is a lack of evidence that providing such 'one-stop shopping' might boost cancer screening, though potential mechanisms such as having a regular source of care have been shown to increase cancer screenings42.
Limitations
This study provides insights capable of informing the development of appropriate interventions to improve rates of breast and cervical cancer screening. Despite this significant contribution, three main limitations exist. First, qualitative inquiry tends to employ relatively small samples. Although capable of providing rich, grounded insights unavailable through pre-existing survey templates, sample sizes and approach limit generalizability. Second, consistent with purposive sampling43, participants were sought who were most likely to fulfill the study aims through their special knowledge of healthcare decisions for underserved Appalachian women, and of Appalachian medical and social services. Given the goal of obtaining the most appropriate data source rather than a representative sample, the authors do not claim that the results represent the perspectives of the entire population. Finally, although the focus on Appalachian residents is warranted due to the health inequities present in this region of the US, study participants were drawn solely from the Appalachian region of the US State of Kentucky, thereby limiting generalization of these findings to the broader US Appalachian population.
Despite potential limitations, the updated and novel insights provided by community members and interpreted in terms of consumer information processing theory emphasize the key role that healthcare providers play in increasing early cancer detection efforts. Greater attention to these recommendations may help reduce the deadly cancer inequities in the Appalachian region of the USA, as well as in other traditionally underserved regions globally.
References
1. Hopenhayn C, Bush H, Christian A, Shelton BJ. Comparative analysis of invasive cervical cancer incidence rates in three Appalachian states. Preventive Medicine 2005; 41(5-6): 859-864.
2. Centers for Disease Control and Prevention. United States cancer statistics: cervical cancer death rates. (Online) 2007. Available: http://apps.nccd.cdc.gov/uscs/ (Accessed 7 November 2011).
3. US Preventive Services Taskforce. Screening for Cervical Cancer. (Online) 2012. Available: http://www.uspreventiveservicestaskforce.org/uspstf/uspscerv.htm (Accessed 22 July 2013).
4. Centers for Disease Control and Prevention. National Breast and Cervical Cancer Early Detection Program. (Online) 2008. Available: http://www.cdc.gov/cancer/nbccedp/ (Accessed 29 January 2010).
5. Blackley D, Behringerb, Zheng S. Cancer Mortality Rates in Appalachia: Descriptive Epidemiology and an Approach to Explaining Differences in Outcomes. Journal of Community Health 2012; 37(4): 804-813.
6. Hendryx M, O'Donnell K, Horn K. Lung cancer mortality is elevated in coal-mining areas of Appalachia. Lung Cancer 2008; 62(1): 1-7.
7. US Preventive Services Taskforce. Screening for Breast Cancer. (Online) 2009. Available: http://www.uspreventiveservicestaskforce.org/uspstf/uspsbrca.htm (Accessed 22 July 2013).
8. Wingo PA, Tucker TC, Jamison PM, Martin H, McLaughlin C, Bayakly R et al. Cancer in Appalachia 2001-2003. Cancer 2008; 112(1): 181-192.
9. Leach CR, Schoenberg NE. The vicious cycle of inadequate early detection: A complementary study on barriers to cervical cancer screening among middle aged and older women. Prevention and Chronic Disease 2007; 4(4): A95.
10. Katz ML, Wewers ME, Single N, Paskett ED. Key Informants' Perspectives Prior to Beginning a Cervical Cancer Study in Ohio Appalachia. Qualitative Health Research 2007; 17(1): 131-141.
11. Schoenberg N, Hopenhayn C, Christian A, Knight EA, Rubio A. An in-depth and updated perspective on determinants of cervical cancer screening among central Appalachian women. Journal of Women and Health 2006; 42(2): 89-105.
12. Hendryx M, Wolfe L, Luo J, Webb B. Self-Reported Cancer Rates in Two Rural Areas of West Virginia with and Without Mountaintop Coal Mining. Journal of Community Health 2012; 37(2): 320-327.
13. Rawl SM, Menon U, Burness A, Breslau ES. Interventions to promote colorectal cancer screening: An integrative review. Nursing Outlook 2012; 60(4): 172-181.
14. Schoenberg NE, Howell B, Fields N. Community strategies to address cancer disparities in Appalachian Kentucky. Family and Community Health 2012; 35(1): 31-43.
15. Drew EM, Schoenberg NE. Deconstructing Fatalism: Ethnographic Perspectives on Women's Decision Making about Cancer Prevention and Treatment. Medical Anthropology Quarterly 2011; 25(2): 164-182.
16. Paskett E, McLaughlin JM, Reiter PL, Lehman AM, Rhoda DA, Katz ML et al. Psychosocial predictors of adherence to risk-appropriate cervical cancer screening guidelines: A cross sectional study of women in Ohio Appalachia participating in the Community Awareness Resources and Education (CARE) project. Preventive Medicine 2011; 50(1-2): 74-80.
17. Shell R, Tudiver F. Barriers to Cancer Screening by Rural Appalachian Primary Care Providers. Journal of Rural Health 2004; 20(4): 368-373.
18. Baron RC, Rimer BK, Coates RJ, Kerner J, Kalra GP, Melillo S et al. Client-Directed Interventions to Increase Community Access to Breast, Cervical, and Colorectal Cancer Screening: A Systematic Review. American Journal of Preventive Medicine 2008; 35(1Suppl): S56-S66.
19. Appalachian Regional Commission. An Analysis of the Financial Conditions of Health Care Institutions in the Appalachian Region and their Economic Impacts. Washington, DC: Appalachian Regional Commission, 2002.
20. Community Preventive Services Task Force. The Community Guide: What Works to Promote Health. (Online) 2012. Available: http://www.thecommunityguide.org/index.html (Accessed 22 July 2013).
21. Appalachian Regional Commission. An Analysis of Disparities in Health Status and Access to Health Care in the Appalachian Region. (Online) 2007. Available: http://www.arc.gov/research/researchreportdetails.asp?REPORT_ID=82 (Accessed 3 May 2012).
22. Centers for Disease Control and Prevention. National Diabetes Surveillance System. (Online) 2011. Available: http://apps.nccd.cdc.gov/DDTSTRS/default.aspx (Accessed 20 June 2012).
23. Pollard K, Jacobson LA. The Appalachian Region: A Data Overview from the 2006-2010 American Community Survey. Washington, DC: Appalachian Regional Commission, 2012.
24. US Agency for Healthcare Research and Quality. United States Preventive Services Task Force Pocket Guide to Preventive Services. (Online) 2012. Available: www.ahrq.gov/clinic/pocketgd.htm‎ (Accessed 23 July 2013).
25. Horner S, Falciglia G, Couch S, Levin L. P33: Assessing Dietary Variety in School-aged Children. Journal of Nutrition Education and Behavior 2007; 39(4): S117-S118.
26. Patton MQ. Qualitative Research and Evaluation Methods, 3rd edn. Thousand Oaks, CA: Sage, 2002.
27. Morse J, Swanson JM, Kuzel AJ. The nature of qualitative evidence. Thousand Oaks, CA: Sage, 2001.
28. Sabatino S, Lawrence B, Elder R, Mercer SL, Wilson KM, DeVinney BM et al. Community Preventive Services Task Force. Effectiveness of interventions to increase screening for breast, cervical, and colorectal cancers: nine updated systematic reviews for The Guide to Community Preventive Services. American Journal of Preventive Medicine 2012; 43(1): 765-786.
29. Chaudhry R, Scheitel SM, McMurtry EK, Leutink DJ, Cabanela RL, Naessens JM et al. Web-Based Proactive System to Improve Breast Cancer Screening: A Randomized Controlled Trial. Archives of Internal Medicine 2007; 167(6): 606-611.
30. Kentucky Cabinet for Health and Family Services. Kentucky Behavioral Risk Factor Surveillance System 2002 Report. Frankfort, KY: Department for Public Health, Division of Epidemiology and Health Planning Surveillance and Health Data Branch, 2002.
31. Acheson LS, Wiesner GL, Zyzanski SJ, Goodwin MA, Stange KC. Family history-taking in community family practice: Implications for genetic screening. Genetics in Medicine 2000; 2(3): 180-185.
32. Bastani R, Maxwell AE, Bradford C, Das IP, Yan KX. Tailored Risk Notification for Women with a Family History of Breast Cancer. Preventive Medicine 1999; 29(5): 355-364.
33. Swanson M, Schoenberg NE, Davis R, Wright S, Dollarhide K. Perceptions of Healthful Eating and Influences on the Food Choices of Appalachian Youth. Journal of Nutrition Education and Behavior 2013; 45(2): 147-53. 35.
34. McBride CM, Emmons KM, Lipkus IM. Understanding the potential of teachable moments: the case of smoking cessation. Health Education Research 2003; 18(2): 156-170.
35. Bellizzi KM, Rowland JH, Jeffery DD, McNeel T. Health Behaviors of Cancer Survivors: Examining Opportunities for Cancer Control Intervention. Journal of Clinical Oncology 2005; 23(34): 8884-8893.
36. Podl TR, Goodwin MA, Kikano GE, Stange KC. Direct observation of exercise counseling in community family practice. American Journal of Preventive Medicine 1999; 17(3): 207-210.
37. Flocke SA, Stange KC. Direct observation and patient recall of health behavior advice. Preventive Medicine 2004; 38(3): 343-349.
38. Roetzheim RG, Christman LK, Jacobsen PB, Schroeder J, Abdulla R, Hunter S. Long-term Results From a Randomized Controlled Trial to Increase Cancer Screening Among Attendees of Community Health Centers. Annals of Family Medicine 2005; 3(2): 109-114.
39. Sallis JF, Owen F, Fisher E. Ecological Models of Health Behavior. In: K Glanz, B Rimer, K Viswanath (Eds); Health Behavior and Health Education: Theory, Research, and Practice. San Francisco, CA: Jossey-Bass, 2008.
40. Israel BA, Eng E, Schulz A, Parker EA. Introduction to Methods in Community-Based Participatory Research for Health. In: BA Israel et al (Eds); Methods in Community-Based Participatory Research for Health. San Francisco, CA: Jossey-Bass, 2005; 3-26.
41. Institute of Medicine. Accelerating Progress in Obestiy Prevention: Solving the Weight of the Nation. Washington, DC: The National Academies Press, 2012.
42. Selvin E, Brett KM. Breast and Cervical Cancer Screening: Sociodemographic Predictors Among White, Black, and Hispanic Women. American Journal of Public Health 2003; 93(4): 618-623.
43. Luborsky M, Rubenstein R. Sampling in qualitative research. Research on Aging 1995; 17(91): 89-113.



