Chagas disease, or American trypanosomiasis, is caused by the protozoan parasite Trypanosoma cruzi. The triatomine bug, also known as the reduviid bug or kissing bug, most commonly transmits the disease via feces. Other modes of transmission include via blood transfusions, vertically from mother to child, through the transplant of solid organs or bone marrow, and rarely through contaminated food or water. T. cruzi is mostly restricted to South America, Central America and parts of North America (Mexico & southern USA)1. According to WHO, approximately 8 million people are currently infected with T. cruzi, most of whom are not aware of the infection2. Rural Latin America is especially at risk due to poor housing conditions favoring vector infestation. Honduras is among the countries with the highest prevalence of T. cruzi infection (3.1%)3. If untreated, infection is lifelong and can be life threatening. About 30% of chronically infected individuals develop irreversible heart damage and roughly 10% experience significant digestive problems2. Once in the chronic phase of the disease, infection is difficult to treat. Additionally, currently available medications carry a high risk of treatment failure and side effects. The cost of Chagas disease treatment is substantial, as well4,5. Ultimately, preventing infection is the best strategy for reducing disease burden.
Over the past 20 years, several vector-control programs, such as the Southern Cone Initiative, Andean Pact Initiative, and Central America Initiative, were implemented in Latin America. Using mass fumigation and housing improvements, some programs were extremely successful in reducing infection rates and risk of infection in several Latin American countries6,7. Blood screening programs and screening of infants born to infected mothers decreased the rate of non-vectorial transmission. Since the launch of vector-control programs, seroprevalence has decreased in Honduras (15.2% in 1980-1985 to 3.1% in 2005). However, the number of individuals at risk of contracting the infection remains high (47% in 1980-1985 vs 49% in 2005)3,8.
Since 2005, Virginia Commonwealth University's Global Health and Health Disparities Program (GH2DP) has partnered with the communities in and around La Hicaca in rural northern Honduras to improve the health of people living in the region. Previous studies within this region showed that disparities in access to health care and disease burden exist within these geographically proximal communities9-12.
Objectives
The purpose of this study was to assess the knowledge, presence of environmental risk factors, and attitudes toward Chagas disease in mountainous yet geographically proximal communities in rural Honduras.
Participant recruitment
A convenience sample of adults receiving care in free medical brigade clinics during May/June 2013 was invited to participate in an anonymous Chagas disease questionnaire. Brigade clinics were held in the towns of La Hicaca and Lomitas in the department of Yoro, Honduras. The clinics served 17 proximal villages, which are under the same local health ministry. They were located in a mountainous region of rural, northern Honduras that was accessible only by four-wheel drive. All clinic attendees aged more than 18 years were offered the opportunity to complete all or part of the questionnaire. Due to low literacy rates, the survey-based questionnaires were verbally administered in Spanish by a member of the healthcare team. Participants received verbal informed consent prior to participation. No incentives were granted for completion of the questionnaire.
Survey
The survey-based questionnaire consisted of 15 multiple-choice questions regarding Chagas disease (Appendix I). Questions were translated from English to Spanish and then back-translated into English to ensure validity. The survey was divided into four sections.
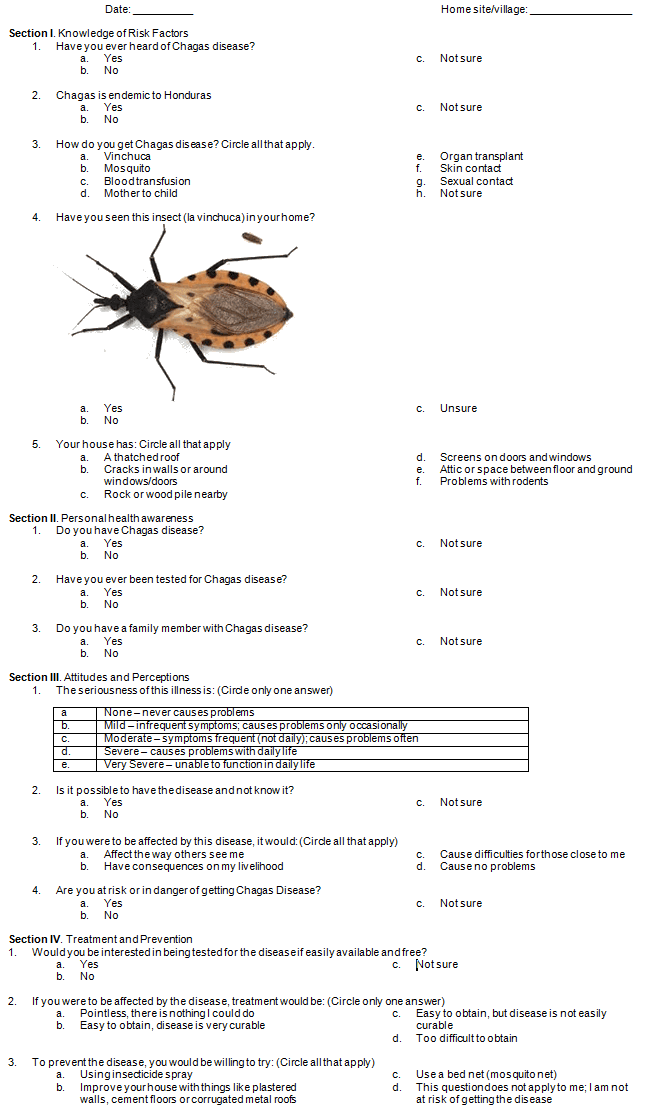
1. Knowledge of Chagas disease risk factors: The first section consisted of five questions that assessed knowledge of Chagas disease risk factors. 'Yes', 'no', and 'not sure' responses were used for three of the questions, asking if people were aware of Chagas disease, if Chagas disease is common in Honduras, and if they had seen the 'kissing bug' in their home. The remaining two questions had multiple-choice answers. Answers to the question of how Chagas disease is transmitted were answered with any combination of the following: 'kissing bug', 'mosquito', 'blood transfusion', 'mother-to-child', 'organ transplant', 'skin contact', 'sexual contact', and 'not sure'. To assess the presence of household risk factors, respondents circled all of the following that were present in their households: 'thatched roof', 'cracks in walls or around windows/doors', 'rock or wood pile nearby', 'screens on doors and windows', and 'problems with rodents'.
2. Personal health awareness: The three questions in this section focused on personal health awareness. The questions were, 'Do you have Chagas disease?', 'Have you ever been tested for Chagas disease?', and 'Do you have a family member with Chagas disease?' All responses used the 'yes', 'no', and 'not sure' format.
3. Attitudes to and perceptions of Chagas disease: The four questions in this section evaluated attitudes and perceptions of Chagas disease. A Likert scale was used to identify the perceived severity of Chagas disease as being 'none', 'mild', 'moderate', 'severe', or 'very severe'. Two questions, 'Is it possible to have Chagas disease and not know it?', and 'Have you ever seen this insect in your home?' (a picture of a 'kissing bug' was provided), had 'yes', 'no', and 'not sure' responses. A multiple-choice question asked what difficulties would be caused if affected by Chagas disease. Respondents chose any combination of the following answers: 'affects the way others see me', 'have consequences on my livelihood', 'cause difficulties for those close to me', and 'cause no difficulties'.
4. Perceptions of treatment and prevention of Chagas disease: Three questions assessed perceptions of treatment and prevention of Chagas disease. The first question assessed interest in being tested for Chagas disease. 'Yes', 'no', and 'not sure' answer choices were given. Respondents chose whether treatment, if affected by the disease, would be 'pointless', 'easy to obtain and disease is very curable', 'easy to obtain, but disease is not easily curable', or 'too difficult to obtain'. The last question of the survey asked what measures people were willing to take in order to prevent the disease. Answer choices were 'use insecticide spray', 'improve your house with things like plastered walls, cement floors or corrugated metal roofs', 'use a mosquito net', and/or 'this question does not apply to me; I am not at risk of getting the disease'.
Statistical analysis
Survey responses were analyzed using SAS statistical software v9.2 (SAS Institute, http://www.sas.com). A descriptive analysis of these data was conducted using frequency counts, and percent response. Based on previous studies showing differences in healthcare access between villages8, it was hypothesized that this study would also show significant differences between communities. Therefore, a two-way χ2 test of significance was used to determine a difference in response between respondents from LH and SV. Fisher's exact test was employed when appropriate based on sample size.
Ethics approval
The institutional review board at Virginia Commonwealth University approved the study protocol (HM14901 VCU Office of Research Subjects Protection).
Out of a convenience sample of 332 clinic attendees, 177 (53%) questionnaires were completed. Sixty-five (37%) participants were from LH, and 112 (63%) were from SV.
Knowledge of Chagas disease risk factors
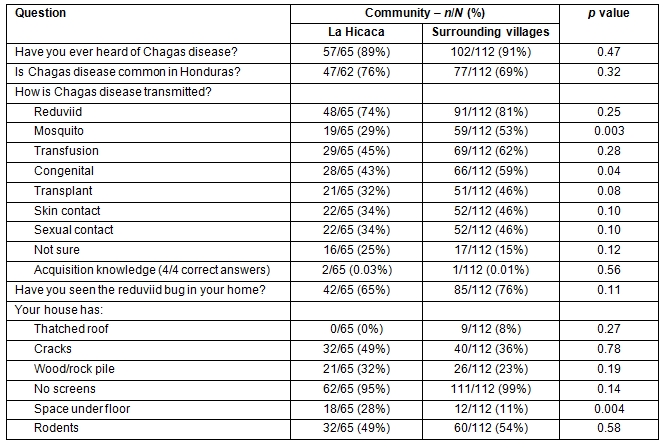
Overall, 159/177 (90%) of respondents were aware of Chagas disease and 124/174 (71%) thought that Chagas disease is common in Honduras. No difference was observed in knowledge of Chagas disease risk factors between LH and SV. Only 3/177 (2%) of respondents demonstrated complete understanding of the transmission of the disease. Complete understanding was defined as marking 'kissing bug', 'blood transfusion', 'mother-to-child', and 'organ transplant' only as modes of Chagas disease transmission. The reduviid bug was seen in 127/177 (72%) of homes. Greater than 99% (n=1) of participants had at least one environmental risk factor for the vector being present in their home. Table 1 summarizes knowledge and environmental risk factors between LH and SV.
Table 1: Knowledge of Chagas disease risk factors
Personal health awareness
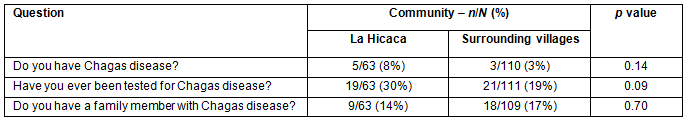
There were no significant differences in personal health awareness observed between villages (Table 2.). While only 8/173 (5%) of people reported having Chagas disease, 134/174 (77%) reported that they had never been tested or were not sure if they had ever been tested for Chagas disease. Only 27/172 (16%) of respondents were aware of a family member with Chagas disease.
Table 2: Personal health awareness of Chagas disease
Attitudes and perceptions
The majority of respondents, 102/126 (81%), felt that Chagas disease is severe or very severe (Fig1). Additionally, 126/171 (74%) thought that it is possible to have the disease and not know it. Only 8/177 (5%) believed that having the disease would cause no problems with their lives or livelihood. The perceived risk of contracting Chagas disease was significantly different between LH and SV. In LH, 15/64 (23%) felt that they were at risk, while 40/106 (38%) in SV felt that they were at risk (p=0.05). Results are summarized in Table 3.
Table 3: Attitudes to and perceptions of Chagas disease
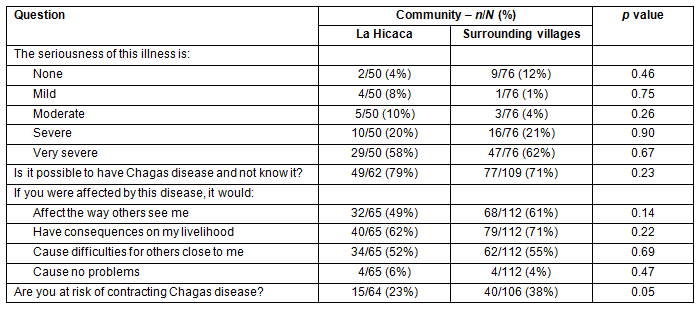
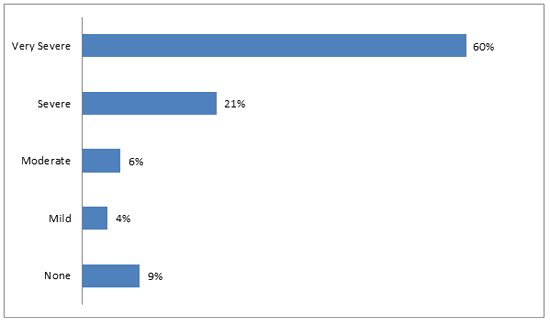
Figure 1: Perceived severity of Chagas disease.
Treatment and prevention
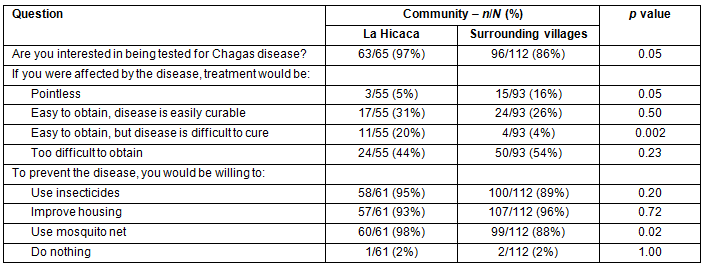
Overall, 159/177 (90%)of survey respondents were interested in being tested for Chagas disease. However, 74/148 (50%) felt that treatment would be too difficult to obtain. There was a significant difference between LH and SV in the number of people that desired testing; 63/65 (97%) in LH, and 96/112 (86%) in SV (p=0.02). In order to prevent contracting the disease, 170/173 (98%) were willing to implement one or more environmental improvements, including the use of insecticides, mosquito nets, and/or housing improvements. Results are summarized in Table 4.
Table 4: Perceptions of Chagas disease treatment and prevention
Discussion
This study assessed knowledge and perceptions of Chagas disease in rural, mountainous Honduras. While all communities surveyed are impoverished, nuances are present in levels of poverty and healthcare pressures. Local data suggest that a stratum of poverty exists between the villages, with LH being the least impoverished (personal communication, Olanchito Ministry of Health, Yoro, Honduras). Although these communities are all mountainous, geographically proximal, share a regional health center, and are under the auspices of the same health ministry, differences in healthcare issues exist. Previous studies performed in this region demonstrated significant differences in healthcare access between LH and nearby communities13. In contrast, this study highlights similarities between LH and SV regarding knowledge of Chagas disease, environmental risk factors, perceptions of the disease, and attitudes toward prevention and treatment. Although awareness of Chagas disease was high, understanding of disease transmission was generally poor. There were no major differences in the presence of environmental risk factors between villages. Furthermore, there was no difference in reported observation of the reduviid bug in respondent homes. There was significant interest in implementing preventative measures across all villages. These results suggest that broadly aimed educational efforts and coordinated environmental improvements would be applicable and relevant across all villages in this geographic area of Honduras.
For greater and longer lasting Chagas disease risk reduction, coordinated and participatory educational and structural improvements are needed. Recently, government interventions, such as spraying of insecticides and health education, were implemented in Latin America to reduce the burden of Chagas disease6,14. Survey responses from the present study suggest that some interventions have been attempted in LH and SV. For example, respondents specified that the local government had performed home fumigation and had replaced many thatched roofs with metal roofs within the last several years. While fumigation temporarily interrupts vector infestation, re-colonization easily occurs under continued poor housing conditions. Cracks in mud walls are a favorite hiding place of the insect. Therefore, a longer lasting solution to control the vector for Chagas disease requires housing improvements, such as replacing mud walls with plaster and floors with cement. A large systematic review looking at multiple Latin American countries found that community participation in vector-control efforts results in improved vector control15. Population-based education about vector behavior and habitat is another important control intervention. For example, many respondents in LH and SV admitted to having a rock or wood pile near their homes, which can serve as a vector breeding place. Simple and consistent educational measures by public health authorities and collaborators, such as removing wood piles alongside dwellings, can reduce the risk of infection. Bed nets are an additional, affordable measure for Chagas disease infection risk reduction.
Some key differences between LH and SV are noteworthy. Respondents from SV perceived a higher risk for Chagas disease. Despite this, fewer people from SV than from LH desired testing for Chagas disease. Reasons for this are unclear, yet may relate to previously reported differences in access to healthcare. Pearson et al. reported in 2012 that inhabitants of SV had less access to health care due to longer travel time, cost, and inability to access transportation. Additionally, respondents from SV were less able to adhere to a healthcare plan13. A perception of greater disease risk and a sense of futility in both disease testing and management may be driven by this lack of access to health care. Additionally, this difference in perceived risk may represent disparities in past Chagas disease outreach efforts between LH and SV.
The body of literature on Chagas disease is vast. However, few articles focus on knowledge and perceptions of Chagas disease in high-risk, Central American communities. To the authors' knowledge, one study specifically reports on knowledge of Chagas disease in Honduras16. The present study, however, is the first in the country to emphasize attitudes toward the disease. Serrano et al. incorporated an assessment of Chagas disease knowledge into a seroepidemiological study in rural Venezuela. Ninety-five percent of those questioned knew that the 'kissing bug' transmits Chagas disease. However, less than 46% of respondents understood Chagas disease transmission and the long-term effects of infection17. Similarly, the present study reports a disparate trend in Chagas disease knowledge, specifically that awareness of Chagas disease is high, while understanding of the disease is poor. Another questionnaire-based study conducted in Argentina revealed a poor understanding of the Chagas disease vector and an inability to describe the mode of transmission18. In contrast, Villela et al. found that knowledge of triatomine insects and of Chagas disease were generally good among a sample of Bambu? adults and children that had previously been exposed to education through the Chagas Disease Control Program19. As in the present study, the majority of respondents felt that Chagas disease is severe.
A recent systematic review of qualitative studies conducted in countries with endemic and non-endemic Chagas disease suggest that social and cultural factors play a substantial role in the emergence and continuation of Chagas disease20. In that review, it was shown that patients with Chagas disease experienced stigmatization and discrimination as a result of their disease. Additionally, people living in Chagas endemic areas associated the disease with death, fear, suffering, distrust, and despair. These elements can influence people's willingness or ability to access health care. These findings highlight the ongoing need for broad educational efforts to dispel misconceptions about the disease. Other observational studies have suggested that social determinants besides knowledge play a large role in behavior change. For example, Chagas disease is a low priority when living conditions are demanding21 . The present study adds to the body of literature on Chagas disease in the Americas and expands the understanding of the structural and educational challenges to Chagas disease control in rural Honduras.
Limitations of the study include the use of convenience sampling methodology and the risk of recall bias by the respondents. Although 53% of adult clinic attendees completed the survey, study participants may not have been representative of the reference population. As all participants came from a similar geographic area of Honduras, the results may not be generalized to other regions of Honduras or Central America. Study strengths include the employment of a structured interview and the use of a standardized questionnaire administered by trained interviewers at the point of care. The questionnaire was culturally sensitive and commensurate with the average educational level of the adult reference population. All data were collected in a uniform manner and inputted into a standardized database, limiting data classification error and bias.
Challenges for limiting the impact of Chagas disease are multiple and widespread. These include structural, socioeconomic and educational hurdles. Short-term, collaborative, medical relief missions play a small yet potentially important role in multi-focal public health interventions. Medical relief teams may collaborate with local officials on focused public health projects, such as education, and structural and sanitary enhancements, to mitigate the risk of infection. In the authors' experience, the findings of this study will guide further collaborative efforts and resource allocation with the local Honduran Health Ministry to reduce the risk of Chagas disease through educational campaigns and targeted interventions, such as continued housing improvements (eg replacing mud walls with plaster and dirt floors with cement, and reallocation of woodpiles), distribution of mosquito nets, and seroprevalence testing, particularly in the communities with the greatest disease-specific knowledge and environmental-structural gaps.
Insights gained from this study are important for understanding the perceptions and attitudes in rural Honduras. A thorough understanding of Chagas disease and its social determinants are increasingly important as a substantial number of Latin Americans continue to migrate to non-endemic countries such as the USA, Canada, Europe, Australia, and Japan. Conversely, geographical variations occur in regards to prevalence, morbidity associated with Chagas disease, and response to treatment22,23. These differences could influence perceptions of the disease and health outcomes. Therefore, it is also important to study each region individually when feasible. In addition, further research is needed to assess the sustained impact of education, fumigation, and housing improvement campaigns. Last, partnerships for the control of Chagas disease require additional assessments to better define the role of short-term medical missions as collaborators with local health authorities. Data from these and similar studies can guide Chagas disease prevention efforts in Central America and rural Honduras.
Despite prior reports of differences in healthcare access across geographically proximal, rural Honduran communities, the present study's findings suggest that knowledge of Chagas disease and environmental risk factors are similar between communities. Chagas disease is believed to be severe by the majority of respondents, yet people from SV reported a higher perceived risk of contracting the disease than those from LH. Understanding of Chagas disease transmission was low across all communities. There was a high level of interest in diagnostic testing and willingness to implement environmental modifications. These findings underscore the need for ongoing and collaborative education and prevention campaigns in the area. In addition, further studies are needed to define the optimal collaborative strategies and partnerships between short term medical relief trips and local health authorities for the control of Chagas disease.
References
1. Rassi A Jr, Rassi A, Marin-Neto JA. Chagas disease. Lancet 2010; 375: 1388-402.
2. WHO. Chagas disease (American trypanosomiasis) fact sheet. (Online) 2013. Available: http://www.who.int/mediacentre/factsheets/fs340/en/index.html (Accessed 26 August 2013).
3. Organización Panamericana de la Salud. Estimacioncuantitativa de la enfermedad de Chagas en las Americas. (In Spanish) Montevideo, Uruguay: Organizacion Panamericana de la Salud, 2006.
4. Castillo-Riquelme M, Guhl F, Turriago B, Pinto N, Rosas F, Florez Martinez M, et al. The costs of preventing and treating Chagas disease in Colombia. PLoS Neglected Tropical Diseases 2008; 2(11): e336.
5. Lee BY, Bacon KM, Bottazzi ME, Hotez PJ. Global economic burden of Chagas disease: a computational simulation model. The Lancet Infectious Diseases 2013; 13(4): 342-348.
6. Moncayo A. Chagas disease: current epidemiological trends after the interruption of vectorial and transfusional transmission in the Southern Cone countries. Memórias do Instituto Oswaldo Cruz 2003; 98(5): 577-591.
7. Hashimoto K, Álvarez H, Nakagawa J, Juarez J, Monroy C, Cordón-Rosales C, et al. Vector control intervention towards interruption of transmission of Chagas disease by Rhodniusprolixus, main vector in Guatemala. Memorias do Instituto Oswaldo Cruz 2012; 107(7): 877-887.
8. WHO Expert Committee on Chagas Disease. Control of Chagas disease: second report of the WHO Expert Committee. World Health Organization Technical Report Series 2002; 905(i-vi): 1-109.
9. Stevens MP, Elam K, Stevens LF, Shodhan S, Markley D, Hemrajani R, et al. Medical needs assessment and infectious diseases concerns in rural Honduras - implications for medical relief planning. International Journal of Infectious Diseases 2010; 14(1): e427.
10. Hemrajani R, Morehouse B, Elam K, Markley D, Stevens LF, Bearman, G et al. Top health concerns in rural Honduras following the introduction of clay water filters. International Journal of Infectious Diseases 2012; 14(1): e66.
11. Stevens MP. Optimizing public health efforts on a medical relief brigade to northern Honduras: the Adult Health Initiative. (Poster) Virginia Public Health Association Second Annual Career Day and Internship Fair, December 2010, Norfolk, VA.
12. Halder GE, Bearman G, Sanogo K, Stevens MP. Water sanitation, access, use and self-reported diarrheal disease in rural Honduras. Rural and Remote Health 13: 2413. (Online) 2013. Available: www.rrh.org.au (Accessed 26 August 2013).
13. Pearson CA, Stevens MP, Sanogo K, Bearman GM. Access and barriers to healthcare vary among three neighboring communities in northern Honduras. International Journal of Family Medicine (Online) 2012. Available: http://www.hindawi.com/journals/ijfm/2012/298472/cta/ (Accessed 26 August 2013).
14. Dias JC, Schofield CJ. The Southern Cone Initiative against Chagas disease. Advances in Parasitology 1999; 42: 1-27.
15. Abad-Franch F, Vega MC, Rolón MS, Santos WS, Rojas de Arias A. Community participation in Chagas disease vector surveillance: systematic review. PloS Neglected Tropical Diseases 2011; 5(6): e1207.
16. Avila Montes G, Martínez Hernández M, Ponce C, Ponce E, Soto Hernández R. Chagas disease in the central region of Honduras: knowledge, beliefs, and practices. (In Spanish). Revista Panamericana de Salud Pública (Pan American Journal of Public Health) 1998; 3(3): 158-163.
17. Serrano O, Mendoza F, Suárez B, Soto A. Seroepidemiología de la enfermedad de Chagas en dos localidades del municipio Costa de Oro, estado Aragua, Venezuela. (In Spanish). Biomédica 2008; 28(1): 108-115.
18. Sanmartino M, Crocco L. Knowledge of Chagas' disease and risk factors in epidemiologically different communities in Argentina. Revista Panamericana de Salud Pública (Pan American Journal of Public Health) 2000; 7(3): 173-178.
19. Villela MM, Pimenta DN, Lamounier PA, Dias JC. Evaluation of knowledge and practices related to Chagas disease and its vectors among adults and children in an endemic region in Minas Gerais State, Brazil. Cad Saude Pública 2009; 25(8): 1701-1710.
20. Ventura-Garcia L, Roura M, Pell C, Posada E, Gascón J, Aldasoro E, et al. Socio-cultural aspects of Chagas disease: a systematic review of qualitative research. PloS Neglected Tropical Diseases 2013; 12;7(9): e2410.
21. Sanmartino M. ¿Qué es lo primero que piensa cuando escucha la palabra 'Chagas'? (In Spanish). Revista de Salud Pública 2009; XIII: 74-78.
22. Coura JR, Borges-Pereira J. Chagas disease. What is known and what should be improved: a systemic review. Revista da Sociedade Brasileira de Medicina Tropical 2012; 45(3): 286-296.
23. Prata A. Evolution of the clinical and epidemiological knowledge about Chagas disease 90 years after its discovery. Memórias do Instituto Oswaldo Cruz 1999; 94(Suppl 1): 81-88.
_____________________________
Appendix I: Survey-based questionnaire on Chagas disease