Approximately a third of Australia's population lives outside urban cities in regional, rural or remote areas1 which cover greater than 85% of Australia's land mass2. The health inequalities experienced by people living in rural and remote communities are well documented3-5. Life expectancy is lower and rates of chronic disease and overall mortality are higher in rural and remote areas5.
The rural environment itself or the effect of 'place'6-8 can have negative and positive effects on the health and wellbeing of people. Place consists of economic, physical, social, environmental and sociocultural factors that interact to define health and influence health behaviours9. Occupational and environmental hazards are more common in rural and remote areas, and the geographical distance between communities can be challenging in terms of health service provision and access to care3,6,10. A review of epidemiological evidence across several developed countries indicated that the impact of 'rural location' likely exacerbated the socioeconomic disadvantage already experienced by many rural communities6, such as lower incomes, education and employment opportunities, which place rural residents at risk of poor health.
However, there are sociocultural benefits from living in rural communities, such as increased social cohesion, connectedness and wellbeing11. Health professionals also report the 'rural lifestyle' and the diversity of rural practice as being important factors that influence them to work and live in rural and remote communities12,13.
The relationship between health and remoteness is particularly important for Aboriginal peoples. More than 65% of the total Aboriginal population live in regional, rural or remote communities compared to metropolitan cities in Australia14. Aboriginal peoples experience significantly poorer health across a range of outcomes than non-Aboriginal people in Australia15. For Aboriginal peoples the connection with country is deeply embedded in culture and has inextricable links with individual and community health and the environment.
The state and territory governments of Australia have aimed to ensure that 'people in rural and remote Australia' are as healthy as other Australians2. However, this vision of health for rural and remote Australians has yet to be realised. Whilst acknowledging that non-communicable diseases contribute a large proportion of the health burden experienced by rural and remote Australians5, previous national data has also demonstrated that rates of communicable disease (eg salmonellosis, pertussis and syphilis) increase with increasing remoteness4.
In addition to health service factors, it is likely that the social determinants of health16, including sociocultural factors and environmental factors, contribute to this differential in communicable disease experienced by rural and remote people in Australia. We sought to conduct a systematic review to summarise the types of studies and subjects of recent research into the epidemiology of communicable disease for rural and remote people in Australia. This review has a particular focus on the social determinants of health in an attempt to provide further understanding of their contribution to the burden of communicable diseases in rural and remote communities and to provide recommendations on research in this field.
Objectives of the review
The review focused on the following questions:
- What areas of research have been conducted over the last decade focusing on communicable diseases in rural and remote communities in Australia? What were the populations of interest, diseases of interest and type of studies conducted?
- From this research, what were the overall findings in relation to the epidemiology of communicable diseases in rural and remote communities of Australia?
- What are the factors that influence the epidemiology of these diseases?
- health service factors - such as surveillance and reporting issues, access to health care, health staffing
- social or cultural factors - such as socioeconomic status, housing and living conditions
- environmental issues - such as contact with animals or contaminated sites.
Definitions and scope
As there is no commonly accepted international definition of what constitutes rural or remote areas, we classified articles based on the geographical location of the main study population in the article, according to the Australian Statistical Geography Classification of Remoteness Categories17. We classified RA 2 (Inner regional) to RA 3 (Outer regional) together as 'rural' and RA 4 (remote) to RA 5 (Very remote) populations together as 'remote' for the purposes of this review2. We used the Department of Health and Ageing (DoHA) online tool to determine the eligibility of studies by remoteness categories for inclusion in our review18. The review included all communicable diseases or diseases with an infectious cause.
Literature review
Data sources and strategy: Potentially eligible peer-reviewed articles were identified by searching articles published in English between June 2004 and June 2013 inclusive in EMBASE, MEDLINE/PubMed, RURAL (Rural and Remote Health Database) and Aboriginal and Torres Strait Islander-Health. An aggregate search of all databases within the Web of Science Core Collection was conducted. For the grey literature, Google and Google Scholar websites were searched and sorted by relevance according to keyword search terms. The first 20 pages retrieved from each website were included for review.
Search terms:
- 'Infectious' OR 'infective' OR 'infection' OR 'notifiable' OR 'communicable disease'; AND
- 'Australia' OR 'Australian'; AND
- 'Rural' OR 'remote' OR 'regional'.
Selection criteria and classification of articles: Articles were then excluded if they described only:
- the burden of chronic or non-communicable diseases
- the epidemiology of communicable diseases in large population groups without a specific focus on rural and/or remote communities
- other public health issues (eg environmental or social issues)
- public health infection control or case management guidelines
- the burden, prevention or management of communicable diseases in plants or animals
- the epidemiology of communicable diseases in rural or remote communities using secondary data or subset analysis only for these groups
- the microbiological, hospital-based clinical diagnostic or environmental epidemiology of communicable diseases in rural remote communities (eg did not focus on the population-level epidemiology of these diseases).
- commentary pieces or perspectives, editorials and conference abstracts.
A data extraction template was developed and the following items were extracted from each article:
- type of article (eg report, journal article, literature review)
- type of study, such as descriptive (ie ecological or cross-sectional study) analytical (ie case-control or cohort study) or experimental study (randomised study designs, etc.) according to WHO criteria19
- study population - rural or remote or both
- type of communicable disease, whether or not it is notifiable and epidemiological trends in diseases studied.
Commentary on the social determinants that were reported in the literature as influencing the epidemiology of the communicable diseases studies were also extracted and a narrative summary is presented in relation to the review questions. Issues over discrepancies concerning analysis of articles were resolved between two authors (EQ and PM) before reporting.
Search strategy results
A total of 2125 articles were retrieved from the five peer-reviewed journal databases and a total of 381 articles were retrieved from the Google website searches (Fig1). After duplicates were excluded, exclusion criteria were applied to the remaining 2287 eligible articles (Table 1); 50 articles20-69 were included for review (Table 2).
Table 1: Summary of excluded articles
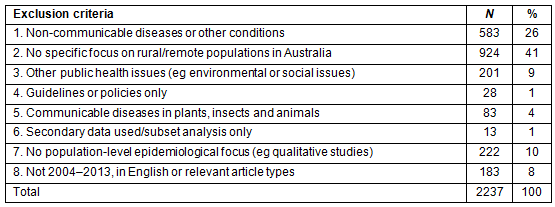 Table 2: Summary of articles included in the review
Table 2: Summary of articles included in the review
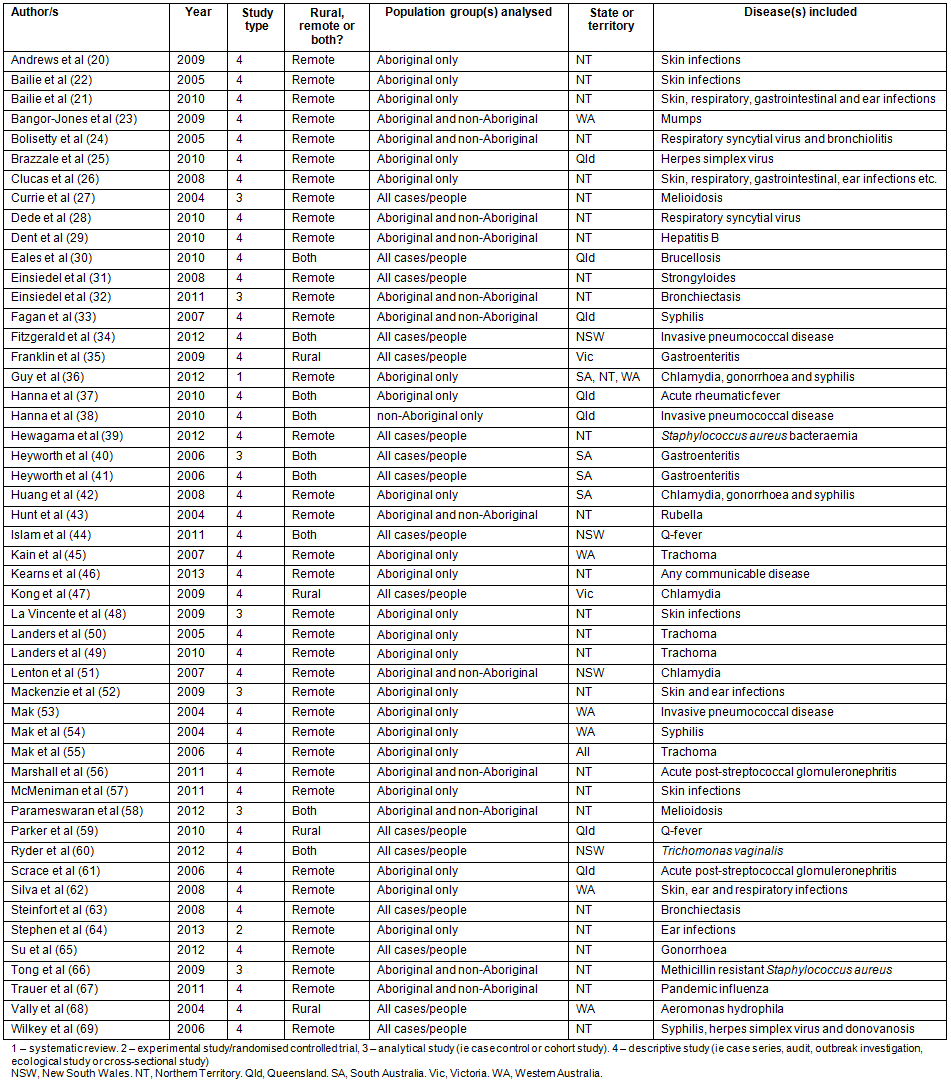
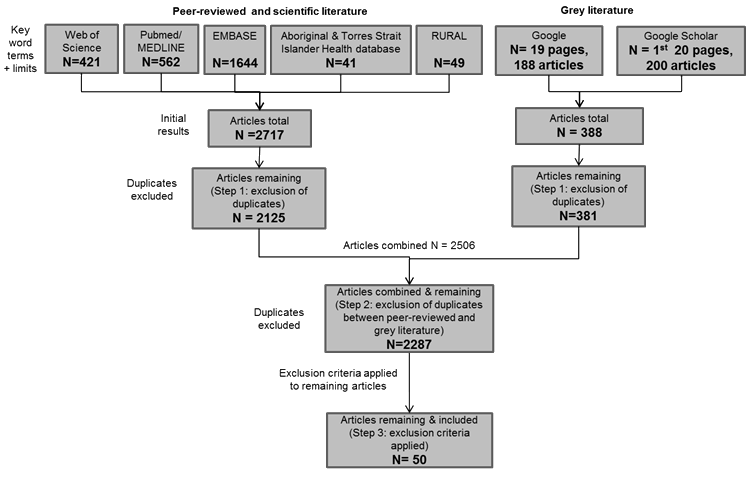 Figure 1: Search strategy algorithm and results.
Figure 1: Search strategy algorithm and results.
Areas and types of research conducted
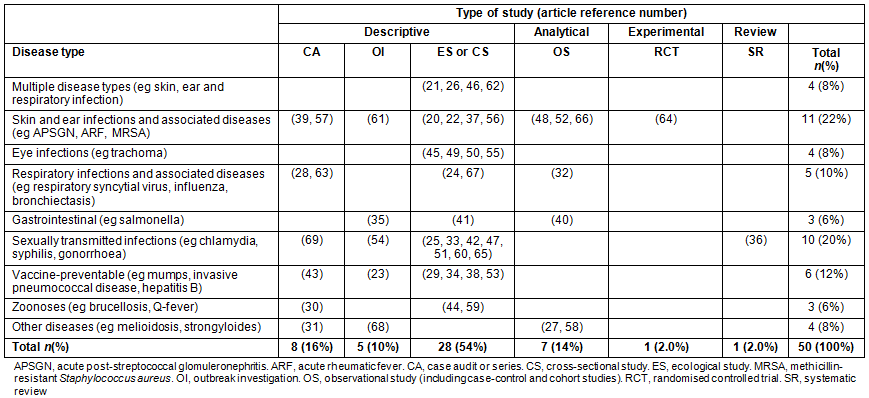
A summary of the 50 articles is provided in Table 1. Of the 50 articles, most (37/50) included only remote and not rural communities, 26/50 were conducted in the Northern Territory (NT) and 21/50 included only Aboriginal peoples. No studies identified and discussed in this review specifically examined the burden of communicable diseases in Torres Strait Islander peoples.
The most commonly investigated groups of communicable diseases were skin and ear infections (n=11 articles)20,22,37,39,48,52,56,57,61,64,66, sexually transmitted infections (n=10 articles)25,33,36,42,47,51,54,60,65,69 and vaccine-preventable diseases (n=6 articles)23,29,34,38,43,53. Other groups of diseases studied included respiratory24,28,32,63,67, eye infections45,49,50, gastroenteritis35,40,41 and zoonoses30,44,59. Strongyloidiasis31 and melioidosis27,58 were also investigated (Table 3).
Nearly all (41/50) of the articles were descriptive studies20-26,28-31,33-35,37-39,41-47,49-51,53-57,59-63,65,67-69, 7/50 were analytical studies27,32,40,48,52,58,66, one article was a systematic literature review on sexually transmitted infections (STIs}36 and one article contained a randomised controlled trial (RCT)64. The area of research related to skin and ear infections and associated diseases contained the only experimental study64 and the most analytical studies48,52,66 included in this review (Table 3).
Quality of studies included
Due to the lack of a singular checklist for assessing the quality of individual studies included in mixed studies reviews70,71, we focused on assessing the reporting quality of the analytical27,32,40,48,52,58,66, RCT64 and systematic review36 articles using separate and commonly used checklists. All seven analytical studies27,32,40,48,52,58,66 included in this review meet all 25 major items of the STROBE72 checklist. The RCT included in this study met 23/25 quality assessment items as listed in the CONSORT checklist73; notable exceptions included specifying who randomised, allocated and followed up on study participants, as well as exact dates defining recruitment and follow-up periods. The systematic review article met 9/11 criteria as included in the AMSTAR checklist74, with improvements warranted for describing the studies excluded as part of the search strategy process. Of the 41 descriptive studies included in this review, 54% (n=28) were ecological or cross-sectional studies (Table 3) with various methods (eg prospective vs retrospective surveys or surveillance reviews), sample sizes (ranging from approximately 40 to >7000) and follow-up periods (ranging from several months to more than a decade). Study type is also noted below to provide an indication of the quality of evidence for each disease area discussed.
Table 3: Summary of articles included in the review - by disease of interest and study type
Epidemiology of communicable diseases in rural and remote areas
Due to the diversity in the measurement and reporting of the epidemiology of communicable diseases, a quantitative synthesis of the articles was not possible. A narrative synthesis of the 48/50 studies is presented below, where the primary objective of the study was to report on the incidence, prevalence, clinical presentation or severity of communicable diseases in rural and/or remote communities.
Multiple disease types: Three descriptive studies reported the epidemiology of multiple disease types (ie skin, respiratory, gastrointestinal and ear infections) in remote Aboriginal children (Table 3). Kearns et al46 reported Aboriginal children presented to health care a median of 21 times in their first year of life (n=320 children), with the vast majority of presentations due to respiratory infections for the period 2001 to 2006. Similarly, Clucas et al26 reported the median number of presentations in a child's first year of life (n=174 children) to be 23; again, respiratory infections were the most common (32%) reason for attending healthcare clinics between 2001 and 2005. The study by Silva et al62 followed up 259 Aboriginal children for the period 1999 to 2005, to determine the impact of the use of a swimming pool on rates of healthcare attendance for common childhood infections (ie skin, ear, respiratory and gastrointestinal infections). The study62 found significant reductions in the rate ratios of skin (-68%), ear (-61%) and respiratory (-52%) infections in one community.
Skin and ear infections and associated diseases: Two descriptive studies20,57, one analytical study48 and one RCT64 examined the epidemiology of skin infections for Aboriginal peoples, predominantly children, living in remote communities (Table 3). Two descriptive studies reported a high prevalence of skin infections in Aboriginal children aged <1 year (>68%)57 and children aged <15 years (greater than 45%)20 in remote communities. The analytical study by La Vincente et al48 examined the effectiveness of scabies treatment in remote Aboriginal households (n=40) from December 2006 to June 2007, revealing that households with complete treatment uptake had a significant five- to six-fold reduction in scabies prevalence.
Stephen et al64 conducted an RCT in 2009 to examine whether 4 weeks of attendance at swimming pools could significantly reduce the prevalence of skin infections in Aboriginal children (n=89) in remote communities and found no significant difference in skin infection or carriage of streptococci for these children.
The analytical study by MacKenzie et al52 investigated differences in age-adjusted incidence of ear infections and ear perforations between two birth cohorts of remote Aboriginal children prior to (1996-2001) and after introduction of pneumococcal vaccination (2001-2004). The study found no significant reduction in odds of ear infections or microbiological ear carriage of Streptococcus pneumoniae vaccine serotypes52.
Three descriptive studies examined the burden of acute post-streptococcal glomerulonephritis56,61 and acute rheumatic fever37 in remote communities, noting the causal links with streptococcal skin or pharyngeal infections. Scrace et al61 conducted an outbreak investigation of acute post-streptococcal glomerulonephritis in children aged <12 years of remote communities of far north Queensland (Qld) during the period February to March 2005 and found that of the 11 confirmed acute post-streptococcal glomerulonephritis cases, 65% were preceded by infected scabies. Acute rheumatic fever became a notifiable disease in Qld during 2004 and although the study by Hanna et al37 revealed an upward trend in the incidence of acute rheumatic fever from 2004 to 2009 for remote Aboriginal peoples, the number of recurrent acute rheumatic fever episodes decreased significantly during this period.
One case audit39 and one analytical study66 examined the epidemiology and clinical presentation of Staphylococcus aureus bacteraemia cases in remote communities of the NT. Hewagama et al39 found an extremely high annual incidence rate of bacteraemia for Aboriginal peoples (160.7/100 000) compared to non-Aboriginal people (8.1/100 000) between 2003 and 2006. Similarly, the prospective matched case-control study by Tong et al66 found a higher incidence of S. aureus bacteraemia (172 cases/100 000 population) in remote Aboriginal peoples compared to the rest of the remote population in the NT from 2006 to 2007 (65 cases/100 000 population).
Eye infections: Four descriptive studies45,49,50,55 analysed the epidemiology of eye infections in remote Aboriginal communities. Two studies49,50 investigated the prevalence of trachoma trichiasis and corneal opacity as complications of recurrent trachoma in remote Aboriginal communities of the NT.
The survey published by Landers et al50 revealed an 8% prevalence of trachoma trichiasis and 3% prevalence of corneal opacity in remote Aboriginal residents (n=181) of the NT in 2003. A similar survey conducted between 2005 and 2008 found that the prevalence of trachoma trichiasis (6.1%) and corneal opacity (3.3%) did not reduce significantly in remote Aboriginal peoples (n=1884) of the NT49, suggesting endemicity of trachoma and its complications in this region.
The study by Kain et al45 measured the prevalence of 'active trachoma'75 in children aged >12 years (n=2975) in remote communities of Western Australia (WA) from 1992 to 2003 and found the prevalence varied widely across these years, but with a significant decline in severe trachoma over time (5% in 1993 to 1% in 2001). Aboriginal children were also greater than 12 times more likely to have active trachoma compared to non-Aboriginal children in this study45. Mak et al55 also found a highly variable but ongoing prevalence of active trachoma (0-40%) in children in remote communities of WA, NT and South Australia (SA) from 1993 to 2007.
Respiratory infections and associated diseases: Two descriptive studies24,28 examined the epidemiology of respiratory syncytial virus in remote communities of the NT. Dede et al28 found significantly higher rates of respiratory syncytial virus in Aboriginal children (29.6/100 000) compared to non-Aboriginal children (10.9/100 000) from 2000 to 2004. Bolisetty et al24 found a very high incidence of respiratory syncytial virus-related hospitalisation during 1998-2000 in children living in remote communities of the NT. A cross-sectional serological survey by Trauer et al67 of residents in the NT revealed a significantly disproportionate infection rate of pandemic H1N1 influenza for Aboriginal people compared to non-Aboriginal people.
Two studies32,63 examined the burden of bronchiectasis in remote residents of the NT. The case audit by Steinfort et al63 of 61 patients presenting to Alice Springs Hospital in the NT for respiratory infections between 2004 and 2005 found that at least 70% of patients had previous recurrent respiratory infections, and human t-cell lymphotropic virus was implicated in 72% of those patients. The retrospective cohort study by Einsiedel et al32 of bronchiectasis admissions to Alice Springs Hospital during 2000-2006 in Aboriginal remote peoples revealed that 60% of cases (n=89) were human t-cell lymphotropic virus-1 seropositive and these adults experienced a significantly higher mortality rate.
Gastrointestinal infections: Three studies analysed the epidemiology of gastroenteritis in children in rural areas of Victoria(Vic)35 and SA40,41. The outbreak investigation of gastroenteritis reported by Franklin et al35 in school children in rural Vic revealed that consumption of untreated private drinking water was the likely source of infection, with microbiological confirmation of Salmonella enterica Typhimurium DT9 in this water supply. In contrast, a cohort study conducted by Heyworth et al40 surveying children 4-6 years (n=1016) about water consumption in rural areas of SA revealed no increased odds of gastroenteritis for children who drank rainwater compared to treated mains water during 6 weeks in the autumn of 1999.
Sexually transmitted infections (STIs): Ten studies25,33,36,42,47,51,54,60,65,69 examined the epidemiology of STIs in a variety of rural and remote settings across a number of states and territories (NT, Qld, NSW, Vic) for different infections (eg syphilis, chlamydia, gonorrhoea, herpes simplex virus). Two descriptive studies33,54 reported a high prevalence of syphilis for remote Aboriginal people. The outbreak investigation by Mak et al54 reported rates of syphilis for young Aboriginal peoples in the Kimberley region to be 439-583/100 000 person years during 2000-2002. A review of notification data from 2001 to 2005 in north Qld by Fagan et al33 found that although the rate of syphilis for Aboriginal peoples decreased significantly from 97/100 000 to 52/100 000, it was still more than 10 times higher than that for non-Aboriginal people.
Two descriptive studies investigated the prevalence of chlamydia in rural Vic47 and remote NSW51. Kong et al47 demonstrated the feasibility of community screening for chlamydia within sporting clubs during 2007, with 709 young adults participating in the program and 5.1% detected as having chlamydia. Lenton et al51 conducted a cross-sectional survey of pregnant women in far west NSW (n=218) between 2004 and 2006 and found that pregnant Aboriginal women were three times more likely to have chlamydia compared to all pregnant women.
Five other descriptive studies investigated the epidemiology of various STIs in remote communities of Qld25, NSW60 and NT42,65,69. Brazzale et al25 found a very high seroprevalence of herpes simplex virus-1 and -2 infection{58-97%) in Aboriginal adults (n=270) in remote areas of the NT between 2007 and 2008. Ryder et al60 found that of the 356 adult women attending a sexual health service in far-west NSW during 2009-2010, 8% were positive for Trichomonas vaginalis. Su et al65 reviewed routine notification data in the NT and found the prevalence of gonorrhoea decreased significantly from 2005 to 2008 in remote adult residents. Wilkey et al69 found that herpes simplex virus-2 was implicated in over half of all cases of genital ulcer disease in remote Aboriginal adults of the NT. The cross-sectional annual prevalence study by Huang et al42 found a significant decrease in the prevalence of chlamydia (67%) and gonorrhoea (58%) from 1996 to 2003.
A recent systematic review by Guy et al36 of STI control programs in the primary healthcare setting in remote Aboriginal communities of Australia demonstrated that well-coordinated and evidence-based programs in SA, NT and WA had significantly decreased rates of STIs (predominantly chlamydia and gonorrhoea) for the review period 1996-2005.
Vaccine-preventable diseases: Six studies examined the epidemiology of vaccine-preventable diseases, such as rubella43, mumps23, hepatitis B29 and invasive pneumococcal disease34,38,53 in remote communities of NT29,43, WA23,53, Qld38 and NSW34. A case audit conducted by Hunt et al43, revealed that antenatal rubella immunity levels were lower for Aboriginal mothers in remote areas of the NT in 1999, leaving women susceptible to this disease during pregnancy.
An outbreak investigation23 of mumps in remote communities of WA from 2007 to 2008 revealed that 92% of all notified cases were Aboriginal people, revealing low vaccination coverage for this group. A serological study by Dent et al29 examining hepatitis B serology of adolescents (n=37) from 1989 to 1990 in remote communities of the NT revealed four had evidence of active infection and under half also had low levels of immunity.
Three studies examined the epidemiology of invasive pneumococcal disease in rural and remote residents of NSW34, Qld38 and WA53. The case series by Mak53 investigated the impact of the 23-valent pneumococcal vaccination program on invasive pneumococcal disease notification rates in Aboriginal and non-Aboriginal remote residents of WA from 2001 to 2005. Invasive pneumococcal disease incidence in Aboriginal peoples significantly declined from 97.8/100 000 person years in 1997 to 38.1/100 000 person years in 200153. Fitzgerald et al34 analysed routine notification data in NSW and found that the annualised rate of invasive pneumococcal disease for all age groups decreased after introduction of the 7-valent invasive pneumococcal disease vaccine in 2005 (from 13.7/100 000 to 8.3/100 000) and that the largest reduction was observed in vaccine serotypes and for children aged <4 years. A similar study conducted by Hanna et al38 in Qld also found significant declines for all ages and vaccine serotypes.
Zoonoses: Three descriptive studies analysed the epidemiology of zoonoses (as listed by the National Notifiable Diseases Surveillance System) in rural and remote communities in NSW44 and Qld30,59. Two studies examined the seroprevalence of Q-fever and found a prevalence of 7%44 and 6.5%59 in rural and remote communities of NSW and Qld respectively. A case audit by Eales et al30 (n=32) for the period 1996-2009 examined the risk factors and clinical presentation of brucellosis and reported that feral pig hunting was indicated as an exposure risk in all cases.
Other diseases: Two studies examined the epidemiology of melioidosis in rural and remote communities of the NT27,58. The prospective cohort study conducted by Currie et al27 from 1989 to 2003 included analysis of 364 cases of melioidosis and found the adjusted relative risk for melioidosis to be three times greater for Aboriginal peoples. The outbreak investigation reported by Parameswaran et al58 of melioidosis cases in the NT between 2009 and 2010 found twice the rate of melioidosis in Aboriginal peoples (102.4/100 000) compared to the overall NT population (50.2/100,000).
Einsiedel31 conducted a case audit of complicated Strongyloides cases in Aboriginal peoples (n=18) in remote communities of the NT and found concurrent human T-lymphotropic virus-1 infection was common and nearly all of the patients died due to sepsis. An outbreak investigation by Vally et al68 revealed cases of Aeromonas were most likely associated with contaminated water, which players were exposed to during a game of mud football.
Factors influencing the epidemiology of communicable diseases in rural and remote areas
Two21,22 of the 50 studies included in this review empirically examined the association between social and environmental factors and rates of communicable diseases in remote Aboriginal communities. The first pilot study by Bailie et al22 prior to 2005 measured the association between poor housing conditions, social conditions and incidence of skin infections in Aboriginal children living in remote communities of the NT. Multivariate analysis of household surveys and primary health care records of 138 children revealed that the most important 'household living practices' influencing the rate of skin infections included a lack of sanitation facilities, four or more children younger than 7 years in the household and low family income22.
The cross-sectional follow-up survey by Bailie et al21 measured the association between housing conditions and social factors and common childhood infections, such as skin, respiratory, ear and gastrointestinal infections, in 328 households in the NT. This study found a strong independent association on multivariate analysis between overall household conditions and respiratory infections21. Significant associations between childhood infections and secondary explanatory variables were also found for skin infections and lack of facilities for household temperature control, gastrointestinal infections and hygienic state of food preparation and storage areas, and ear infections and childcare attendance21. Around half of all the remaining papers discussed multiple factors that could influence the epidemiology of communicable diseases in rural and remote communities (Table 4).
Table 4: Summary of reported factors influencing the epidemiology of communicable diseases in rural and remote communities
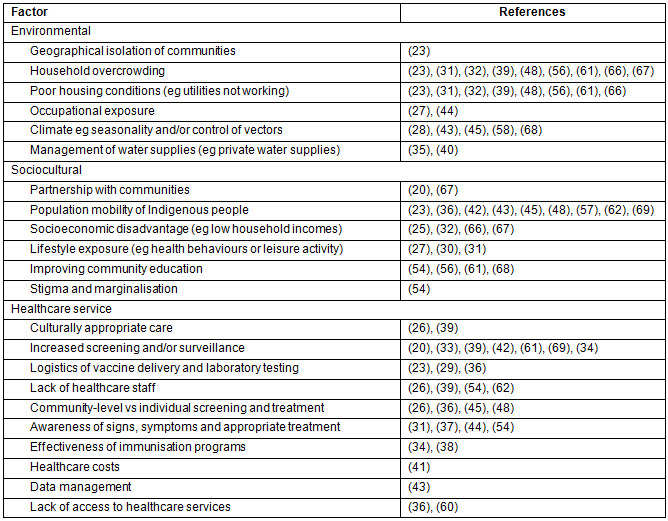
Health service factors: Health service factors were discussed in 26 articles20,23,26,29,31-34,36-39,41-45,48,54,60-62,65,66,69 as influencing the epidemiology of communicable diseases in rural and remote areas. Increased or enhanced screening and surveillance were discussed in eight articles20,33,34,39,42,61,65,69 as being important to ensure cases are detected early and treatment programs remain targeted. Other factors included difficulties with health service delivery due to lack of staff in remote areas26,39,54,62 and the need for continued medical education of the presentation and treatment for some infectious diseases31,37,44,54,66,68.
Sociocultural factors: Sociocultural factors were discussed in 21 articles20,23,25,27,30-32,36,42,43,48,54,56,57,61,62,66-69, including the population mobility of Aboriginal people23,36,42,43,48,57,62,69 along with socioeconomic disadvantage25,32,66,67. It is worth highlighting that only two articles20,67 explicitly discussed the importance of community partnerships in designing and/or implementing epidemiological or research studies designed to reduce the burden of communicable diseases.
Environmental factors: Environmental factors were discussed in 18 articles23,27,28,31,32,35,39,40,43-45,48,56,58,61,66-68, in particular household overcrowding23,31,32,39,48,56,61,66,67 and poor housing conditions23,31,32,39,48,56,61,66,67. The climate28,43,45,58,68 was also discussed by several authors as being an important factor determining the incidence of some communicable diseases with seasonal patterns or in relation to the logistics of vaccine delivery being difficult in remote communities of Australia.
Discussion
This systematic review of the published literature over the last decade showed a substantial increased burden of communicable diseases in remote Aboriginal communities of Australia, in particular skin, eye and respiratory infections in children. Rates of skin pathogen-associated diseases (eg acute post-streptococcal glomerulonephritis, acute rheumatic fever and bacteraemia) were high amongst Aboriginal peoples compared to non-Aboriginal people in remote communities. Evidence suggests that improvements have been made in reducing the burden of STIs in remote Aboriginal communities. Evidence has been emerging to demonstrate that poor housing conditions are associated with an increased risk of skin, ear, respiratory and gastrointestinal infections in children. There is also growing recognition of the role of sociocultural and environmental factors in influencing the epidemiology of communicable diseases in rural and remote communities. However, further investment into higher quality community-based research that addresses the social determinants of the burden of communicable diseases in remote communities of Australia is needed.
Our review of the literature identified mostly descriptive studies examining the epidemiology of diseases in remote areas of Australia, predominantly for Aboriginal peoples. Very few studies reported on the epidemiology of communicable diseases in rural communities only or for non-Aboriginal populations in general. There was also a lack of research investigating the epidemiology of zoonoses (in the narrow sense) and tropical diseases despite the evidence suggesting an increased risk for rural and remote residents.
The focus in the published literature on the disease burden for Aboriginal peoples in remote communities is particularly relevant and important given the need to address the health gap between Aboriginal peoples and non-Aboriginal people15 and the increasing differential in health status associated with remoteness in Australia3. This review has also identified a substantial burden of non-notifiable communicable diseases for remote Aboriginal peoples over the past 10 years, particularly skin, eye and respiratory infections for Aboriginal children in remote communities.
Whilst health service factors were the most commonly discussed factors identified in this review there was also clear recognition that overcrowding in households, poor housing conditions in remote Aboriginal communities in particular, and other socioeconomic detriments, can place people at increased risk for communicable diseases. Only two empirical studies were identified in this review. Both demonstrated an association between housing and living conditions and rates of common childhood infections (eg skin, ear and respiratory infections)21,22. The Housing for Health program, which now operates in many parts of Australia, is a practical example of how surveying and then fixing and maintaining adequate housing conditions for remote communities can reduce the rates of common infectious diseases76. The evaluation of the program in NSW revealed an overall 38% reduction in hospitalisation for infectious diseases in Aboriginal communities participating in the program, compared to the rest of the rural NSW Aboriginal population without the program76.
There is increasing acknowledgement of the need to take a social determinants approach to designing interventions and strategies that reduce the burden of communicable diseases77-79. Significant advances in the past century have reduced the burden of communicable diseases for most groups in the population, for example through vaccination, enhanced surveillance systems for improved detection and a more timely response, as well as more effective treatments to reduce morbidity and mortality from communicable diseases. However, as detected in this review, some population groups, such as remote Aboriginal children, remain vulnerable to communicable diseases through a combination of health service and social and environmental factors.
The findings from this review suggest that there may still be room for improvement in non-experimental epidemiological studies describing the communicable disease burden in rural and remote areas. Examples include recruiting and analysing larger samples over longer time periods and including analysis of risk factors that may help explain any changes in disease incidence or prevalence over time.
This review also highlighted that more analytical or experimental evidence is required to better demonstrate which strategies or interventions targeting the social determinants of health might be effective at reducing the burden of communicable diseases for rural and remote communities. However, the challenges with conducting this type of research cannot be underestimated80. Some of these challenges may include the need to select the best correlates or indicators of the social determinants and health outcomes to monitor over time and the number or type of intervening variables that can ultimately affect the dose relationship between intervention strategy and health outcomes. In addition, the difficulties with smaller populations in having sufficient power for intervention studies, the vast distances between communities, as well as issues with conducting large studies over a long timeframe, make the design of these intervention studies challenging in terms of trying to demonstrate impact.
Despite the methodological difficulties mentioned above, addressing the social determinants of communicable diseases in rural and remote communities of Australia is a public health priority that will require intersectoral action and multicomponent public health programs. Frameworks81 or tools82 that assist public health practitioners with the design or conduct of these studies will help promulgate this type of research. Epidemiological or research studies that include community-based partnerships83 can also be an important way to empower local communities to address locally relevant public health issues (eg including community-based views in the design of services or programs or training local community members and/or health staff to help deliver enhanced services).
Limitations
Given the difficulties associated with keyword searching and comprehensively searching the grey literature, we will not have identified every eligible article for review; however, our review process was systematic and covered a decade. The possible pathogen-related cause or development of some non-communicable diseases was not a focus of this review. Also, the published literature only provides an insight into the true burden of communicable diseases experienced by rural and remote communities of Australia.
Communicable diseases remain an important contributor to preventable morbidity in rural and remote communities of Australia, particularly for Aboriginal peoples. There is growing recognition of the role of sociocultural and environmental factors in contributing to this burden of disease. Overall, there is a lack of high-level evidence demonstrating which strategies or interventions might be the most effective at alleviating the social determinants of the communicable disease burden experienced by rural and remote communities. Further investment into higher quality community-based research that aims to provide an epidemiological and social understanding of the burden of disease in order to develop evidence-based prevention and control strategies is needed.
References
1. Australian Bureau of Statistics. 3218.0 - Regional population growth, Australia, 2011. Australian Bureau of Statistics. (Online) 2011. Available: http://www.abs.gov.au/ausstats/abs@.nsf/Products/3218.0~2011~Main+Features~Main+Features?OpenDocument. (Accessed 2 February 2014).
2. Australian Health Minister's Conference Standing Council on Health. National Strategic Framework for Rural and Remote Health. (Online) 2012. Available: http://www.ruralhealthaustralia.gov.au/internet/rha/publishing.nsf/Content/NSFRRH-homepage (Accessed 2 February 2014).
3. Australian Institute of Health and Welfare. Rural, regional and remote health: indicators of health status and determinants of health. Rural Health Series no. 9, cat. no. PHE 97. Canberra, ACT: AIHW, 2008.
4. Australian Institute of Health and Welfare. Rural, regional and remote health- information framework and indicators. Version 1b. Cat. no. PHE 69. Canberra, ACT: AIHW, 2005.
5. Australian Institute of Health and Welfare. Australia's health 2010. Australia's Health series. No. 12, cat. no. AUS 122. Canberra, ACT: AIHW, 2010.
6. Smith KB, Humphreys JS, Wilson MG. Addressing the health disadvantage of rural populations: how does epidemiological evidence inform rural health policies and research? Australian Journal of Rural Health 2008; 16(2): 56-66.
7. Wakerman J. Rural and remote public health in Australia: building on our strengths. Australian Journal of Rural Health 2008; 16(2): 52-55.
8. Dixon J, Welch N. Researching the rural-metropolitan health differential using the 'social determinants of health'. Australian Journal of Rural Health 2000; 8(5): 254-260.
9. Macintyre S, Ellaway A, Cummins S. Place effects on health: how can we conceptualise, operationalise and measure them? Social Science and Medicine 2002; 55(1): 125-139.
10. Australian Institute of Health and Welfare. Rural, regional and remote health: indicators of health system performance. No. 10, cat. no. PHE 103. Canberra, ACT: AIHW, 2008.
11. Ziersch AM, Baum F, Darmawan IG, Kavanagh AM, Bentley RJ. Social capital and health in rural and urban communities in South Australia. Australian and New Zealand Journal of Public Health 2009; 33(1): 7-16.
12. Humphreys JS, Jones MP, Jones JA, Mara PR. Workforce retention in rural and remote Australia: determining the factors that influence length of practice. Medical Journal of Australia 2002; 176(10): 472-476.
13. Campbell N, McAllister L, Eley D. The influence of motivation in recruitment and retention of rural and remote allied health professionals: a literature review. Rural Remote Health 12: 1900. (Online) 2012. Available: www.rrh.org.au (Accessed 12 July 2014).
14. Australian Bureau of Statistics. 3238.0.55.001 - Estimates of Aboriginal and Torres Strait Islander Australians, June 2011. (Online) 2013. Available: http://www.abs.gov.au/ausstats/abs@.nsf/mf/3238.0.55.001. (Accessed 16 July 2014).
15. Australian Health Ministers Advisory Council. Aboriginal and Torres Strait Islander Health Performance Framework. Canberra, ACT: AHMAC, Department of Health and Ageing, 2012.
16. Beard JR, Tomaska N, Earnest A, Summerhayes R, Morgan G. Influence of socioeconomic and cultural factors on rural health. Australian Journal of Rural Health 2009; 17(1): 10-15.
17. Australian Bureau of Statistics. ASGC Remoteness Classification: purpose and use. (Online) 2013. Available: http://www.abs.gov.au/websitedbs/d3110122.nsf/0/f9c96fb635cce780ca256d420005dc02/$FILE/Remoteness_Paper_text_final.pdf. (Accessed 2 February 2014).
18. Department of Health and Ageing. DoctorConnect [Internet database]. Available: http://www.doctorconnect.gov.au/locator (Accessed 13 October 2013).
19. World Health Organization. Basic epidemiology, 2nd edn. Geneva, Switzerland: WHO, 2006.
20. Andrews RM, Kearns T, Connors C, Parker C, Carville K, Currie BJ, et al. A regional initiative to reduce skin infections amongst aboriginal children living in remote communities of the Northern Territory, Australia. PLoS Neglected Tropical Diseases 2009; 3(11): e554.
21. Bailie R, Stevens M, McDonald E, Brewster D, Guthridge S. Exploring cross-sectional associations between common childhood illness, housing and social conditions in remote Australian Aboriginal communities. BMC Public Health 2010; 10: 147.
22. Bailie RS, Stevens MR, McDonald E, Halpin S, Brewster D, Robinson G, et al. Skin infection, housing and social circumstances in children living in remote Indigenous communities: testing conceptual and methodological approaches. BMC Public Health 2005; 5: 128.
23. Bangor-Jones RD, Dowse GK, Giele CM, Van Buynder PG, Hodge MM, Whitty MM. A prolonged mumps outbreak among highly vaccinated Aboriginal people in the Kimberley region of Western Australia. Medical Journal of Australia 2009; 191(7): 398-401.
24. Bolisetty S, Wheaton G, Chang AB. Respiratory syncytial virus infection and immunoprophylaxis for selected high-risk children in Central Australia. Aust Journal of Rural Health 2005; 13(5): 265-270.
25. Brazzale AG, Russell DB, Cunningham AL, Taylor J, McBride WJH. Seroprevalence of herpes simplex virus type 1 and type 2 among the Indigenous population of Cape York, Far North Queensland, Australia. Sexual Health 2010; 7(4): 453-459.
26. Clucas DB, Carville KS, Connors C, Currie BJ, Carapetis JR, Andrews RM. Disease burden and health-care clinic attendances for young children in remote Aboriginal communities of northern Australia. Bulletin of the World Health Organization 2008; 86(4): 275-281.
27. Currie BJ, Jacups SP, Cheng AC, Fisher DA, Anstey NM, Huffam SE, et al. Melioidosis epidemiology and risk factors from a prospective whole-population study in northern Australia. Tropical Medicine and International Health 2004; 9(11): 1167-1174.
28. Dede A, Isaacs D, Torzillo PJ, Wakerman J, Roseby R, Fahy R, et al. Respiratory syncytial virus infections in Central Australia. Journal of Paediatrics and Child Health 2010; 46(1-2): 35-39.
29. Dent E, Selvey CE, Bell A, Davis J, McDonald MI. Incomplete protection against hepatitis B among remote Aboriginal adolescents despite full vaccination in infancy. Communicable Diseases Intelligence 2010; 34(4): 435-439.
30. Eales KM, Norton RE, Ketheesan N. Short report: brucellosis in Northern Australia. American Journal of Tropical Medicine and Hygiene 2010; 83(4): 876-878.
31. Einsiedel L, Fernandes L. Strongyloides stercoralis: a cause of morbidity and mortality for indigenous people in Central Australia. Internal Medicine Journal 2008; 38(9): 697-703.
32. Einsiedel L, Fernandes L, Spelman T, Steinfort D, Gotuzzo E. Bronchiectasis is associated with Human T-Lymphotropic virus 1 infection in an Indigenous Australian population. Clinical Infectious Diseases 2011; 54(1): 43-50.
33. Fagan PS, Cannon FM. Syphilis in remote north Queensland. Communicable Diseases Intelligence 2007; 31(1): 125-127.
34. Fitzgerald T, Masseya PD, Islama F. Changes in invasive pneumococcal disease serotypes in a regional area of Australia following three years of 7vPCV introduction. Western Pacific Surveillance and Response Journal 2012; 3(2): 1-6.
35. Franklin LJ, Fielding JE, Gregory J, Gullan L, Lightfoot D, Poznanski SY, et al. An outbreak of Salmonella Typhimurium 9 at a school camp linked to contamination of rainwater tanks. Epidemiology and Infection 2009; 137(3): 434-440.
36. Guy R, Ward JS, Smith KS, Su JY, Huang RL, Tangey A, et al. The impact of sexually transmissible infection programs in remote Aboriginal communities in Australia: a systematic review. Sexual Health 2012; 9(3): 205-212.
37. Hanna JN, Clark MF. Acute rheumatic fever in indigenous people in North Queensland: some good news at last? Medical Journal of Australia 2010; 192(10): 581-584.
38. Hanna JN, Humphreys JL, Murphy DM, Smith HV. Invasive pneumococcal disease in non-Indigenous people in north Queensland, 2001-2009. Medical Journal of Australia 2010; 193(7): 392-396.
39. Hewagama S, Spelman T, Einsiedel LJ. Staphylococcus aureus bacteraemia at Alice Springs Hospital, Central Australia, 2003-2006. Internal Medicine Journal 2012; 42(5): 505-512.
40. Heyworth J, Glonek G, Maynard E, Baghurst P, Finlay-Jones J. Consumption of untreated tank rainwater and gastroenteritis among young children in South Australia. International Journal of Epidemiology 2006; 35(4): 1051-1058.
41. Heyworth JS, Jardine A, Glonek G, Maynard EJ. Incidence, impact on the family and cost of gastroenteritis among 4 to 6-year-old children in South Australia. Journal of Gastroenterology and Hepatology 2006; 21(8): 1320-1325.
42. Huang RL, Torzillo PJ, Hammond VA, Coulter ST, Kirby AC. Epidemiology of sexually transmitted infections on the Anangu Pitjantjatjara Yankunytjatjara Lands: results of a comprehensive control program. Medical Journal of Australia 2008; 189(8): 442-445.
43. Hunt JM, Lumley J. Top end rural and remote Indigenous women: an Australian population group vulnerable to rubella. Communicable Diseases Intelligence 2004; 28(4): 499-503.
44. Islam A, Ferguson J, Givney R, Graves S. Short report: seroprevalence to Coxiella burnetii among residents of the Hunter New England region of New South Wales, Australia. American Journal of Tropical Medicine and Hygiene 2011; 84(2): 318-320.
45. Kain S, Morgan W, Riley D, Dorizzi K, Hogarth G, Yu DY. Prevalence of trachoma in school children of remote Western Australian communities between 1992 and 2003. Clinical and Experimental Ophthalmology 2007; 35(2): 119-123.
46. Kearns T, Clucas D, Connors C, Currie BJ, Carapetis JR, Andrews RM. Clinic attendances during the first 12 months of life for Aboriginal children in five remote communities of Northern Australia. PLoS One 2013; 8(3): e58231.
47. Kong FYS, Hocking JS, Link CK, Chen MY, Hellard ME. Sex and sport: chlamydia screening in rural sporting clubs. BMC Infectious Diseases 2009; 9(73).
48. La Vincente S, Kearns T, Connors C, Cameron S, Carapetis J, Andrews R. Community management of endemic scabies in remote Aboriginal communities of Northern Australia: low treatment uptake and high ongoing acquisition. Plos Neglected Tropical Diseases 2009; 3(5): e444.
49. Landers J, Henderson T, Craig J. Prevalence and associations of blinding trachoma in indigenous Australians within central Australia: the Central Australian Ocular Health Study. Clinical and Experimental Ophthalmology 2010; 38(4): 398-404.
50. Landers J, Kleinschmidt A, Wu J, Burt B, Ewald D, Henderson T. Prevalence of cicatricial trachoma in an indigenous population of Central Australia: the Central Australian Trachomatous Trichiasis Study (CATTS). Clinical and Experimental Ophthalmology 2005; 33(2): 142-146.
51. Lenton JA, Freedman E, Hoskin K, Knight V, Turley D, Balding B, et al. Chlamydia trachomatis infection among antenatal women in remote far west New South Wales, Australia. Sexual Health 2007; 4(2): 139-140.
52. Mackenzie GA, Carapetis JR, Leach AJ, Morris PS. Pneumococcal vaccination and otitis media in Australian Aboriginal infants: comparison of two birth cohorts before and after introduction of vaccination. BMC Pediatrics 2009; 9(1).
53. Mak DB. Invasive pneumococcal disease in the Kimberley, 1995-2001. Australian Journal of Rural Health 2004; 12(6): 237-240.
54. Mak DB, Johnson GH, Plant AJ. A syphilis outbreak in remote Australia: epidemiology and strategies for control. Epidemiology and Infection 2004; 132(5): 805-812.
55. Mak DB, O'Neill LM, Herceg A, McFarlane H. Prevalence and control of trachoma in Australia, 1997-2004. Communicable Diseases Intelligence 2006; 30(2): 236-247.
56. Marshall CS, Cheng AC, Markey PG, Towers RJ, Richardson LJ, Fagan PK, et al. Acute post-streptococcal glomerulonephritis in the Northern Territory of Australia: a review of 16 years data and comparison with the literature. The American Journal of Tropical Medicine and Hygiene 2011; 85(4): 703-710.
57. McMeniman E, Holden L, Kearns T, Clucas DB, Carapetis JR, Currie BJ, et al. Skin disease in the first two years of life in Aboriginal children in East Arnhem Land. Australasian Journal of Dermatology 2011; 52(4): 270-273.
58. Parameswaran U, Baird RW, Ward LM, Currie BJ. Melioidosis at Royal Darwin Hospital in the big 2009-2010 wet season: comparison with the preceding 20 years. Medical Journal of Australia 2012; 196(5): 345-348.
59. Parker N, Robson J, Bell M. A serosurvey of Coxiella burnetii infection in children and young adults in South West Queensland. Australian and New Zealand Journal of Public Health 2010; 34(1): 79-82.
60. Ryder N, Woods H, McKay K, Giddings N, Lenton JA, Little C, et al. Trichomonas vaginalis prevalence increases with remoteness in rural and remote New South Wales, Australia. Sexually Transmitted Diseases 2012; 39(12): 938-941.
61. Scrace M, Koko K. An outbreak of acute post-streptococcal glomerulonephritis in remote Far North Queensland. Australian Journal of Rural Health 2006; 14(4): 160-163.
62. Silva DT, Lehmann D, Tennant MT, Jacoby P, Wright H, Stanley FJ. Effect of swimming pools on antibiotic use and clinic attendance for infections in two Aboriginal communities in Western Australia. Medical Journal of Australia 2008; 188(10): 594-598.
63. Steinfort DP, Brady S, Weisinger HS, Einsiedel L. Bronchiectasis in Central Australia: a young face to an old disease. Respiratory Medicine 2008; 102(4): 574-578.
64. Stephen AT, Leach AJ, Morris PS. Impact of swimming on chronic suppurative otitis media in Aboriginal children: a randomised controlled trial. Medical Journal of Australia 2013; 199(1): 51-55.
65. Su JY, Condon JR. Trends in testing and notification for genital gonorrhoea in a northern Australian district, 2004-2008. Sexual Health 2012; 9(4): 384-388.
66. Tong SY, Bishop EJ, Lilliebridge RA, Cheng AC, Spasova-Penkova Z, Holt DC, et al. Community-associated strains of methicillin-resistant Staphylococcus aureus and methicillin-susceptible S. aureus in indigenous Northern Australia: epidemiology and outcomes. Journal of Infectious Diseases 2009; 199(10): 1461-1470.
67. Trauer JM, Laurie KL, McDonnell J, Kelso A, Markey PG. Differential effects of pandemic (H1N1) 2009 on remote and indigenous groups, Northern Territory, Australia, 2009. Emerging Infectious Diseases 2011; 17(9): 1615-1623.
68. Vally H, Whittle A, Cameron S, Dowse GK, Watson T. Outbreak of Aeromonas hydrophila wound infections associated with mud football. Clinical Infectious Diseases 2004; 38(8): 1084-1089.
69. Wilkey JE, Fethers KA, Latif AS, Kaldor JM. Genital ulcer disease in central Australia: predictors of testing and outcomes. Sexual Health 2006; 3(2): 119-122.
70. Shamliyan T, Kane RL, Dickinson S. A systematic review of tools used to assess the quality of observational studies that examine incidence or prevalence and risk factors for diseases. Journal of Clinical Epidemiology 2010; 63(10): 1061-1070.
71. Sanderson S, Tatt ID, Higgins JP. Tools for assessing quality and susceptibility to bias in observational studies in epidemiology: a systematic review and annotated bibliography. International Journal of Epidemiology 2007; 36(3): 666-676.
72. Noah N. The STROBE initiative: STrengthening the Reporting of OBservational studies in Epidemiology (STROBE). Epidemiology and Infection 2008; 136(7): 865.
73. Schulz KF, Altman DG, Moher D, Group C. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMC Medicine 2010; 8: 18.
74. Shea BJ, Grimshaw JM, Wells GA, Boers M, Andersson N, Hamel C, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Medical Research Methodology 2007; 7: 10.
75. Thylefors B, Dawson CR, Jones BR, West SK, Taylor HR. A simple system for the assessment of trachoma and its complications. Bulletin of the World Health Organization 1987; 65(4): 477-483.
76. Aboriginal Environmental Health Unit. Closing the gap: 10 years of housing for health in NSW. An evaluation of a healthy housing intervention. North Sydney, NSW: Population Health Division, NSW Health, 2010.
77. Braveman P. Accumulating knowledge on the social determinants of health and infectious disease. Public Health Reports 2011; 126 Suppl 3: 28-30.
78. Semenza JC, Giesecke J. Intervening to reduce inequalities in infections in Europe. American Journal of Public Health 2008; 98(5): 787-792.
79. Semenza JC, Suk JE, Tsolova S. Social determinants of infectious diseases: a public health priority. European Communicable Disease Bulletin 2010; 15(27): 2-4.
80. Braveman P, Egerter S, Williams DR. The social determinants of health: coming of age. Annual Review of Public Health 2011; 32: 381-398.
81. Thomas SB, Quinn SC, Butler J, Fryer CS, Garza MA. Toward a fourth generation of disparities research to achieve health equity. Annual Review of Public Health 2011; 32: 399-416.
82. McDonald EL, Bailie R, Michel T. Development and trialling of a tool to support a systems approach to improve social determinants of health in rural and remote Australian communities: the healthy community assessment tool. International Journal for Equity in Health 2013; 12: 15.
83. Israel BA, Coombe CM, Cheezum RR, Schulz AJ, McGranaghan RJ, Lichtenstein R, et al. Community-based participatory research: a capacity-building approach for policy advocacy aimed at eliminating health disparities. American Journal of Public Health 2010; 100(11): 2094-2102.


