Skin tumors are the most frequent malignancy worldwide among all malignant neoplasms. Between 2 and 3 million new cases of non-melanoma skin cancers and 132 000 new cases of melanoma are diagnosed annually1. According to the Brazilian National Cancer Institute, 175 760 new cases of non-melanoma skin cancers are expected in 20162.
Among the tumors of the skin, non-melanoma represents more than 90% of cases. Most of these lesions are associated with low mortality rates, but they can cause disfigurement and sequelae due to the treatment, since the lesions predominantly appear in skin areas exposed to the sun, like the face, neck and arms3,4. However, when detected at an early stage, the therapeutic approach is accessible and inexpensive, with low morbidity3,5.
Usually the skin tumors do not invade lymphatic vessels and the metastasis rates are less than 1%. However, if undiagnosed and untreated for a long period of time these lesions can invade areas such as eyes, peripheral muscle, nerves, bone and cartilage6-8. In contrast, melanoma has a lower incidence (2-5% of skin cancers), but is responsible for most deaths due to its high metastatic potential and drug refractory characteristic9 ; also, its prognosis is closely related to the clinical stage at diagnosis10,11. Although skin cancer is the most common neoplasm in tropical countries12, there is no population screening for skin cancer in Brazil. For an efficient skin cancer screening, an examination of the all of the skin on the body is expected2,13. Moreover, the cost-effectiveness of skin cancer screening programs in decreasing morbidity or mortality is still controversial2,14,15.
Dermatology as well as radiology and pathology specialties have been using telemedicine16, among other things, for health care in remote areas and in populations with limited access to healthcare providers17. Early in 1995, dermatologists started to use telemedicine resources in a rural area of the state of Oregon in the USA18.
When used as a tool in the identification and monitoring lesions in large populations, teledermatology has proved to be potentially important in reduction of medical costs and in reducing the time taken to identify suspicious lesions19,20. Reports based on the use of digital photography of skin lesions remotely scanned and sent to specialized medical centers, where qualified professionals evaluated them, showed very impressive results in the diagnosis of skin cancer21,22.
Developing countries, in which there are huge areas with an important deficit of physicians, could benefit from teledermatology, because it would allow early diagnosis of malignant lesions of the skin by training nurses to identify likely malignant lesions and send the skin photographic images to an experienced dermatologist23-26 for evaluation.
A clinical questionnaire for patients in addition to medical images is an accepted way to improve the reading of images by radiologists27-29. Similarly, telemedicine is used in cardiology: electrocardiogram images are sent with clinical data for remote medical assistance29.
In 1970, a study in England showed that in 80% of outpatients the isolated clinical questionnaire was responsible for 82.5% of diagnoses, clinical examination for more than 8.75% and additional tests for more than 8.75%30. Several other studies showed that the main diagnostic tool of the physician is a clinical questionnaire and physical examination31,32.
A movement began in the 1990s for the implementation of a rigorous scientific methodology for diagnostic studies. This initiative states that the clinical questionnaire and physical examination give the doctor all the necessary tools for diagnosis, diagnostic hypotheses, and to identify patients in the early stages of diseases that could lead to death33.
Developing countries with large geographical areas usually have insufficient medical assistance as well as an imbalanced distribution of doctors. The use of teledermatology could be of benefit for the remote regions of these countries, allowing early diagnosis of skin cancer in this population. One possible strategy is to train local healthcare providers to take pictures of suspicious skin lesions and send the images for remote diagnosis by experienced dermatologists34,35.
The aim of this study was to evaluate the importance of the increment of a clinical patient questionnaire to digital photography for the diagnosis of skin cancer via teledermatology in distant and rural areas of Brazilian territory using mobile units.
The Cancer Prevention Department (CDP) and the Department of Skin Cancer at Barretos Cancer Hospital (BCH, Barretos, Brazil), performed the skin cancer screening. The program is based on the use of Mobile Unit (MU) of Cancer Prevention that regularly visits the remote areas of Brazil. This MU is fully equipped to perform clinical procedures and small surgical procedures; regarding skin lesions, the team is able to perform 40 clinical dermatology examinations or procedures per day, including cryotherapy and surgery. One well-trained medical doctor is in charge of skin lesion examination by visual inspection.
A nurse from the local municipality who was trained previously at BCH screened all patients examined at the MU. A more detailed description of the MU concept has been published previously36. Such local nurses are trained to identify suspicious lesions for skin cancer by BCH nurses and by a physician from BCH with experience in skin cancer screening. A day of training is offered specifically to exemplify the different types of the more frequent skin lesions, and didactic materials (eg image CDs, banners, leaflets and brochures) are given to support the educational training. The nurses and physician from BCH are also responsible for speaking with the clinicians in the municipalities to convince them about the importance of their participation in the prevention activities and also for closely assisting the local nurses' triage. In addition, they are encouraged to principally invite the underprivileged people that live in the suburbs or in rural areas and to inform them that all of the procedures related to the cancer prevention and treatment are free36.
Study participants
A total of 623 patients referred to the MU appointment participated in the study; 436 were part of the first phase of the study and 186 in the second phase as described below.
First phase: A nurse from a location that MU would visit screened patients for skin malignancy from February 2010 to July 2011. At the MU appointment, clinical assessment, diagnosis (benign or malignant), all the photography and biopsies of lesions in cases of suspected of skin malignancy were performed by a medical doctor. At this point, nurses were present to assist the medical procedures. All the biological material collected (biopsies or surgical specimen) was properly identified, stored in bottles with 10% formalin and sent to the BCH pathology department for analysis.
The photographic images were obtained from a distance of approximately 60 cm from the skin lesion in order to assess the topographic localization of the lesion, and a second image was captured as close as possible to assess the details of the lesion. If the visual inspection suggested a neoplastic lesion, the medical doctor performed the excision of the lesions.
Second phase: Patients were screened (by a nurse) between June and July 2012 in the same manner as for the first phase; however, a specific questionnaire (not applied in the first phase) was applied after the medical visit and the photography was performed.
The two photos of each lesion were done in the following way: the first was taken from a long distance; the second was taken near the injury using the macro function in the same way as in the first phase. Briefly, the clinical information collected were race, previous history of skin cancer, previous history of familial cancer, previous or current history of skin disease, physical characteristics of the lesion, duration of the symptoms and evolution (growing and/or changing aspect), anatomical localization of the skin lesion, macroscopic characteristics of the lesion (pigmented, elevated, ulcerated, infiltrative, cutaneous horn-like, edges regular or irregular), if the lesion is recurrent, if the patient was previously submitted to radiotherapy at the site of the skin lesion, if the patient had been or still was in contact with risk factors at work (chemical, sunlight exposition), use of sunscreen, history of tanning, use of immunosuppressive drugs, type of skin phototype recorded (A (I and II), B (III and IV), C (V and VI)).
Digital photography
The camera used was a Sony Cybershot DSC-S780, of resolution 8.1 megapixels. The lighting photography place was a clinic room and four fluorescent lamps of 54 V each were used to illuminate the photographic field. The camera used was the same between the first and second phases in order to avoid any bias of image resolution.
All photos were stored in JPEG format, and they were properly identified and sent blindly, via internet, to two BCH medical doctors with at least 10 years of experience in diagnosis and treatment of skin cancer.
The clinical questionnaire was sent with the photos in the second phase of the study.
Evaluation of photographs
Physicians assessed the photos independently and made the diagnosis classifying them as (1) lesion suspicious for malignancy (e.g. carcinoma, Bowen's disease, keratoacanthomas), (2) lesion with benign features (such as simple warts, ringworm, simple nevi and other skin conditions that do not need to be referred to a cancer hospital) or (3) not evaluated lesion (due to insufficient image for diagnosis - photograph of poor quality (low light, high reflection or out of focus) or ill-defined lesion). Teledermatology non-concordant lesions were invited for new clinical evaluation and submitted to surgery (when necessary).
The same doctor was present in the two phases of the study. Figure 1 presents a flowchart of the process of this study.
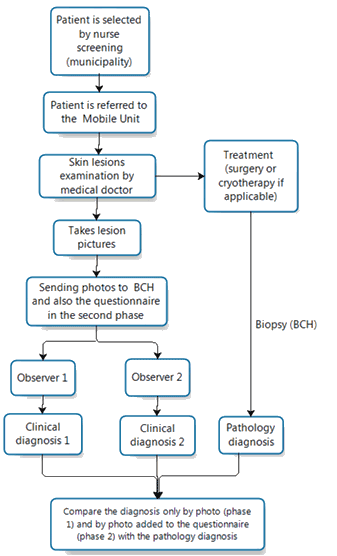
Figure 1: Flowchart of the study.
Statistic evaluation
The gold standard to calculate the sensitivity, specificity, positive and negative predictive values and accuracy of observers was the pathology report. These results were compared and submitted to descriptive statistical analysis and accuracy, and a receiver operating characteristic (ROC) curve was constructed. For this, the Statistical Package for the Social Sciences v19.0 (IBM Corporation; http://www.spss.com) and R v3.2.3 were used (https://www.r-project.org/). The R package pROC (http://web.expasy.org/pROC/) was used to compute the DeLong's test for two ROC curves comparison. Statistical significance (p value) adopted was 0.05.
Ethics approval
The study was approved by the Research Ethics Committee of BCH, with protocol number 377/2010, in accordance with the ethical standards by the initial 1964 Declaration of Helsinki and including its later amendments. Consent was obtained before starting the study.
All patients undergoing surgical procedures, cases of extensive lesions or anatomical areas of difficult surgical access were referred to BCH for the appropriate procedures (such as surgery and/or radiotherapy). Patients with benign lesions were referred for dermatological treatment in the municipality.
The mean age was 63.1 years (standard deviation=13.1) and 61.7 years (standard deviation=14.2) for patients of phase 1 and 2 respectively. In both phases, most patients had clear skin, skin type (according to Fitzpatrick Scale37) I and II (77.2% and 61.2%), most malignant lesions were located in the region of the face and head and neck (70.3% and 63.7%). The vast majority of lesions were diagnosed at an early stage, 0 or 1 (89.5% and 84.1%). The majority of lesions, were classified as basocellular carcinoma (68.3% and 64%), and melanoma represented 1.4% and 2.2%, respectively, of skin cancers. The pathological results of the first and second phases are shown in Table 1.
The photographs of skin lesions were assessed by medical doctors of BCH (observer 1 and 2) for diagnosis. The sensitivity, specificity, positive and negative predictive values and accuracy are shown in Table 2.
Comparison of the two phases shows that the ROC curves for the observers increased significantly (observer 1: p=0.003; observer 2: p=0.002) (Figs 2,3).
Table 1: Pathological results for phases 1 and 2
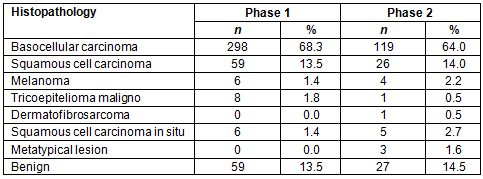
Table 2: Observer's performance for phases 1 and 2
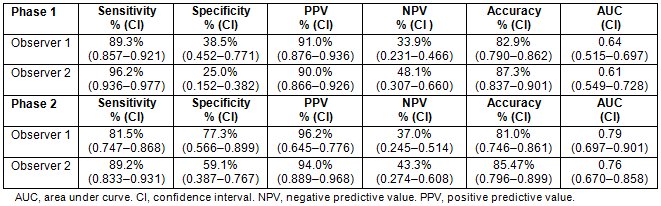
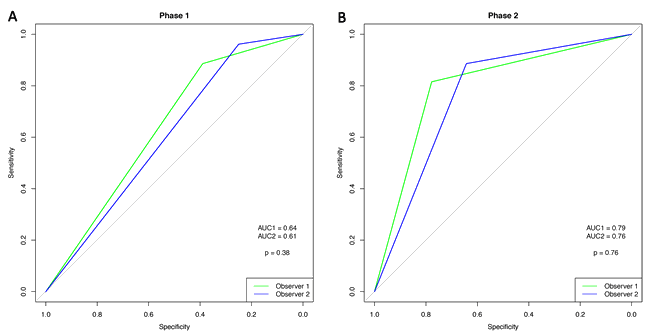
Figure 2: Receiver operating characteristic curve for phase 1 (A) and phase 2 (B).
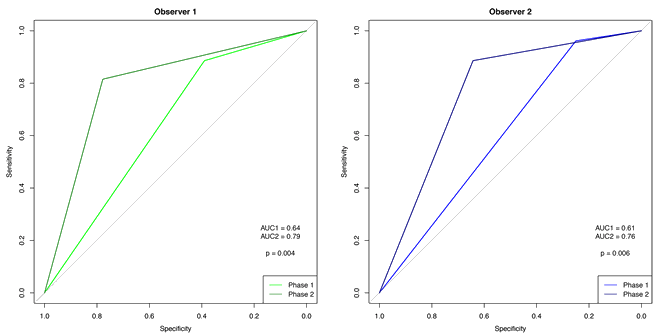
Figure 3: Receiver operating characteristic curve for observers 1 and 2.
Discussion
Brazil experiences strong solar radiation in most parts of its vast territory. This constitutes a risk factor for skin cancer. Optimizing the options for skin lesion diagnosis is critical to reduce bad outcomes in populations affected by skin cancer that live and work in rural remote areas. The present study's results have demonstrated that teledermatology has major potential to detect early lesions of skin cancer and also to favor the recognition of a skin lesion.
Currently, melanoma and non-melanoma skin cancer screening programs do not exist in Brazil; consequently, diagnosis is often postponed and the lesions are diagnosed tardily and mainly at an advanced stage. Non-melanoma tumors are associated with extended local resections, which are in turn associated with high morbidity38. Skin melanoma has a worse prognosis, leading to potential loss of significant years of life with indirect costs associated with morbidity and premature mortality39.
Screening of skin cancer has been implemented in several countries, showing the potential to reduce cases of advanced cancers at diagnosis40.
In Germany a recent study observed that melanoma mortality decreased by 47% among men and 49% among women after implementation of screening for skin cancer, providing significant evidence that screening can prevent a reasonable number of deaths from melanoma. However, despite the strong favorable evidence, the data lack definitive arguments to support the statement that the skin cancer screening program reduced melanoma mortality in the Schleswig-Holstein area40.
Access to specialized medical services in remote areas or even in the outskirts of large cities is a recurrent problem in many parts of the world. Additionally, there is an evident difficulty in convincing skilled medical doctors to work in rural and remote areas of both developed36,41,42 and developing countries43. For these reasons the authors strongly advocate the use of teledermatology to aid in reducing the distance between patients and medical doctors specialized in dermatology44, reducing the time to perform the diagnosis and indicate adequate treatment when compared to the traditional healthcare system24,45,46. Although there are few studies using teledermatology in screening skin cancer, they showed encouraging results24,26 . In this context the use of teledermatology in skin cancer prevention looks promising.
The images of the lesions are the main semiotic element of dermatological diagnosis. The effectiveness of teledermatology and dermatological telescreening depends on the quality of the picture, clinical representativeness of the lesion, significance of the lesion aspects inherent in three dimensions, palpation characteristics and dimension of the lesions, as well as notions about the topography of the injuries47.
Following these premises, the two-dimensional format of the digital images certainly represents limitations regarding the estimated size, topography and palpation consistency of the lesions; however, the present study's results were satisfactory in relation to the histopathological findings, and a clinical questionnaire improved the sensitivity and accuracy of observers.
Due to patients having been previously screened by the physician of the MU there were a small number of negative cases; consequently, specificity and negative predictive values were low in the first and second phases. However, there was a slight increase in the negative predictive value in the second phase. Due to this limitation of the study this result cannot be attributed to the use of the questionnaire; however, the ROC curves for the observers increased significantly between the two study phases.
The study data revealed an exciting perspective for further programs of skin cancer prevention. If the questionnaire had been introduced at the establishment of these activities the results herein may have been more remarkable. Additionally, the design of this study did not permit the evaluation of false negative results.
The clinical questionnaire combined with digital photography has increased the performance of observers in opportunistic skin cancer screening. Teledermatology proved to be an effective tool to improve population screening for patients at high risk of skin cancer.
Acknowledgements
The authors thank the Public Ministry of Labor (Research, Prevention and Education of Occupational Cancer) in Campinas, SP, Brazil, and the Allini Mafra da Costa from Research and Support Department of BCH for their assistance in preparing the data for statistical analyses.
References
1. World Health Organization. Ultraviolet radiation: global solar UV index. Fact sheet no. 271. Geneva: World Health Organization, 2009.
2. Instituto Nacional de Câncer. Estimativa 2016: incidência de câncer no Brasil. Rio de Janeiro: Ministério da Saúde. Available: http://www.inca.gov.br/wcm/dncc/2015/estimativa-2016.asp (Accessed February 2016).
3. Lee EH, Klassen AF, Lawson JL, Cano SJ, Scott AM, Pusic AL. Patient experiences and outcomes following facial skin cancer surgery: a qualitative study. Australasian Journal of Dermatology 2015; 57(3): 100-104. http://dx.doi.org/10.1111/ajd.12323
4. Schuch AP, Yagura T, Makita K, Yamamoto H, Schuch NJ, Agnez-Lima LF, et al. DNA damage profiles induced by sunlight at different latitudes. Environmental and Molecular Mutagenesis 2012; 53(3): 198-206. http://dx.doi.org/10.1002/em.21678
5. Kim RH, Armstrong AW. Nonmelanoma skin cancer. Dermatologic Clinics 2012; 30(1): 125-139. http://dx.doi.org/10.1016/j.det.2011.08.008
6. Ministério da Saúde, Instituto Nacional de Câncer José Alencar Gomes da Silva, Coordenação Geral de Ações Estratégicas, Coordenação de Prevenção e Vigilância. Estimativa 2012: incidência de câncer no Brasil. Rio de Janeiro: Instituto Nacional de Câncer, 2011.
7. Mehta KS, Mahajan VK, Chauhan PS, Sharma AL, Sharma V, Abhinav C, et al. Metastatic basal cell carcinoma: a biological continuum of basal cell carcinoma? Case Reports in Dermatological Medicine 2012; 10(5): 297-315. http://dx.doi.org/10.1155/2012/157187
8. Hofmann-Wellenhof R, Pellacani G, Malvehy J, Soyer HP. Reflectance confocal microscopy for skin diseases. Berlin: Springer Science & Business Media, 2012. http://dx.doi.org/10.1007/978-3-642-21997-9
9. Livingstone E, Zimmer L, Vaubel J, Schadendorf D. Current advances and perspectives in the treatment of advanced melanoma. Journal of the German Society of Dermatology 2012; 10(5): 319-325. http://dx.doi.org/10.1111/j.1610-0387.2012.07895.x
10. Barth A, Wanek LA, Morton DL. Prognostic factors in 1,521 melanoma patients with distant metastases. Journal of the American College of Surgeons 1995; 181(3): 193-201.
11. Miller AJ, Mihm MC, Jr. Melanoma. New England Journal of Medicine 2006; 355(1): 51-65. http://dx.doi.org/10.1056/NEJMra052166
12. Lomas A, Leonardi?Bee J, Bath?Hextall F. A systematic review of worldwide incidence of nonmelanoma skin cancer. British Journal of Dermatology 2012; 166(5): 1069-1080. http://dx.doi.org/10.1111/j.1365-2133.2012.10830.x
13. McDonald CJ. American Cancer Society perspective on the American College of Preventive Medicine's policy statements on skin cancer prevention and screening. CA: A Cancer Journal for Clinicians 1998; 48(4): 229-231. http://dx.doi.org/10.3322/canjclin.48.4.229
14. Smith RA, Manassaram-Baptiste D, Brooks D, Cokkinides V, Doroshenk M, Saslow D, et al. Cancer screening in the United States, 2014: a review of current American Cancer Society guidelines and current issues in cancer screening. CA: A Cancer Journal for Clinicians 2014; 64(1): 30-51. http://dx.doi.org/10.3322/caac.21212
15. DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, et al. Cancer treatment and survivorship statistics, 2014. CA: A Cancer Journal for Clinicians 2014; 64(4): 252-271. http://dx.doi.org/10.3322/caac.21235
16. Perednia DA, Brown NA. Teledermatology: one application of telemedicine. Bulletin of the Medical Library Association 1995; 83(1): 42-47.
17. Moffatt J, Eley D. Barriers to the up-take of telemedicine in Australia: a view from providers. Rural and Remote Health (Internet) 2011; 11(1): 1581. Available: www.rrh.org.au (Accessed January 2016).
18. Perednia DA, Brown N. Teledermatology: one application of telemedicine. Bulletin of the Medical Library Association 1995; 83(1): 42.
19. Byamba K, Syed?Abdul S, García?Romero M, Huang CW, Nergyi S, Nyamdorj A, et al. Mobile teledermatology for a prompter and more efficient dermatological care in rural Mongolia. British Journal of Dermatology 2015; 173(1): 265-267. http://dx.doi.org/10.1111/bjd.13607
20. Bergmo TS. An economic model of remote specialist consultations using videoconferencing. International Journal on Advances in Life Sciences 2015; 7(1,2): 39.
21. Wurm EM, Hofmann?Wellenhof R, Wurm R, Soyer HP. Telemedicine and teledermatology: past, present and future. Journal of the German Society of Dermatology 2008; 6(2): 106-112. http://dx.doi.org/10.1111/j.1610-0387.2007.06440.x
22. Trindade MA, Wen CL, Neto CF, Escuder MM, Andrade VL, Yamashitafuji TM, et al. Accuracy of store-and-forward diagnosis in leprosy. Journal of Telemedicine and Telecare 2008; 14(4): 208-210. http://dx.doi.org/10.1258/jtt.2008.071203
23. Silveira C, Silva T, Fregnani J, da Costa Vieira R, Haikel R, Syrjanen K, et al. Digital photography in skin cancer screening by mobile units in remote areas of Brazil BMC Dermatology 2014; 14(1): 1.
24. Moreno-Ramirez D, Ferrandiz L, Nieto-Garcia A, Carrasco R, Moreno-Alvarez P, Galdeano R, et al. Store-and-forward teledermatology in skin cancer triage: experience and evaluation of 2009 teleconsultations. Archives of Dermatology 2007; 143(4): 479-484. http://dx.doi.org/10.1001/archderm.143.4.479
25. Oliveira MR, Wen CL, Neto CF, Silveira PS, Rivitti EA, Böhm GM. Web site for training nonmedical health-care workers to identify potentially malignant skin lesions and for teledermatology. Telemedicine Journal and e-Health 2002; 8(3): 323-332. http://dx.doi.org/10.1089/15305620260353216
26. Moreno-Ramirez D, Ferrandiz L, Bernal AP, Duran RC, Martin JJ, Camacho F. Teledermatology as a filtering system in pigmented lesion clinics. Journal of Telemedicine and Telecare 2005; 11(6): 298-303. http://dx.doi.org/10.1258/1357633054893364
27. Zennaro F, Oliveira Gomes JA, Casalino A, Lonardi M, Starc M, Paoletti P, et al. Digital radiology to improve the quality of care in countries with limited resources: a feasibility study from Angola. PloS One 2013; 8(9): e73939. http://dx.doi.org/10.1371/journal.pone.0073939
28. Bashshur RL, Shannon GW, Smith BR, Alverson DC, Antoniotti N, Barsan WG, et al. The empirical foundations of telemedicine interventions for chronic disease management. Telemedicine and e-Health 2014; 20(9): 769-800. http://dx.doi.org/10.1089/tmj.2014.9981
29. Ribeiro AL, Alkmim MB, Cardoso CS, Carvalho GG, Caiaffa WT, Andrade MV, et al. Implementation of a telecardiology system in the state of Minas Gerais: the Minas Telecardio Project. Arquivos Brasileiros de Cardiologia 2010; 95(1): 70-78. http://dx.doi.org/10.1590/S0066-782X2010005000060
30. Hampton J, Harrison M, Mitchell J, Prichard J, Seymour C. Relative contributions of history-taking, physical examination, and laboratory investigation to diagnosis and management of medical outpatients. British Medical Journal 1975; 2(5969): 486-489. http://dx.doi.org/10.1136/bmj.2.5969.486
31. Sandler G. The importance of the history in the medical clinic and the cost of unnecessary tests. American Heart Journal 1980; 100(6): 928-931. http://dx.doi.org/10.1016/0002-8703(80)90076-9
32. Roshan M, Rao A. A study on relative contributions of the history, physical examination and investigations in making medical diagnosis. Journal of the Association of Physicians of India. 2000; 48(8): 771-775.
33. Sackett DL, Rennie D. The science of the art of the clinical examination. Journal of the American Medical Association 1992; 267(19): 2650-2652. http://dx.doi.org/10.1001/jama.1992.03480190092040
34. Shapiro M, James WD, Kessler R, Lazorik FC, Katz KA, Tam J, et al. Comparison of skin biopsy triage decisions in 49 patients with pigmented lesions and skin neoplasms: store-and-forward teledermatology vs face-to-face dermatology. Archives of Dermatology 2004; 140(5): 525-528. http://dx.doi.org/10.1001/archderm.140.5.525
35. Moreno-Ramirez D, Ferrandiz L, Nieto-Garcia A, Carrasco R, Moreno-Alvarez P, Galdeano R, et al. Store-and-forward teledermatology in skin cancer triage: experience and evaluation of 2009 teleconsultations. Archives of Dermatology 2007; 143(4): 479-483. http://dx.doi.org/10.1001/archderm.143.4.479
36. Mauad EC, Silva TB, Latorre MR, Vieira RA, Haikel RL, Vazquez VL, et al. Opportunistic screening for skin cancer using a mobile unit in Brazil. BMC Dermatology 2011; 11(1): 12. http://dx.doi.org/10.1186/1471-5945-11-12
37. Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Archives of Dermatology 1988; 124: 869-871. http://dx.doi.org/10.1001/archderm.1988.01670060015008
38. Fundação Oncocentro de São Paulo. Acesso ao Banco de Dados - Registro Hospital de Câncer. (Internet) 2011. Available: http://200.144.1.68/cgi-bin/dh?rhc/rhc-geral.def (Accessed 19 December 2012).
39. Katalinic A, Waldmann A, Weinstock MA, Geller AC, Eisemann N, Greinert R, et al. Does skin cancer screening save lives?: an observational study comparing trends in melanoma mortality in regions with and without screening. Cancer 2012; 118(21): 5395. http://dx.doi.org/10.1002/cncr.27566
40. Choudhury K, Volkmer B, Greinert R, Christophers E, Breitbart EW. Effectiveness of skin cancer screening programmes. British Journal of Dermatology 2012; 167(Suppl 2): 94-98. http://dx.doi.org/10.1111/j.1365-2133.2012.11091.x
41. Dumont JC ZP, Church J, Thi CL. International mobility of health workforce management in Canada: myths and realities. OECD Health working paper. France: OECD iLibrary, 2008. http://dx.doi.org/10.1787/228478636331
42. Matsumoto M, Inoue K, Kajii E, Takeuchi K. Retention of physicians in rural Japan: concerted efforts of the government, prefectures, municipalities and medical schools. Rural and Remote Health (Internet) 2010; 10(2): 1432. Available: www.rrh.org.au (Accessed January 2016).
43. Mollahaliloglu S, Aydogan U, Kosdak M, Oncul HG, Dilmen U. Physician scarcity in underdeveloped areas of Turkey: what do new graduate physicians think? Rural Remote Health (Internet) 2012; 12: 2067. Available: www.rrh.org.au (Accessed January 2016).
44. Bryld LE, Heidenheim M, Dam TN, Dufour N, Vang E, Agner T, et al. Teledermatology with an integrated nurse-led clinic on the Faroe Islands - 7 years' experience. Journal of the European Academy of Dermatology and Venereology 2011; 25(8): 987-990. http://dx.doi.org/10.1111/j.1468-3083.2010.03884.x
45. Diepgen TL. Occupational skin diseases. Journal of the German Society of Dermatology 2012; 10(5): 297-315. http://dx.doi.org/10.1111/j.1610-0387.2012.07890.x
46. Ferrandiz L, Moreno-Ramirez D, Nieto-Garcia A, Carrasco R, Moreno-Alvarez P, Galdeano R, et al. Teledermatology-based presurgical management for nonmelanoma skin cancer: a pilot study. Dermatological Surgery 2007; 33(9): 1092-1098. http://dx.doi.org/10.1097/00042728-200709000-00009
47. High WA, Houston MS, Calobrisi SD, Drage LA, McEvoy MT. Assessment of the accuracy of low-cost store-and-forward teledermatology consultation. Journal of the American Academy of Dermatology 2000; 42(5 Pt 1): 776-783. http://dx.doi.org/10.1067/mjd.2000.104519
Modified on 22 June 2017: the correction "The third author, Adriane Feijó Evangelista, was initially omitted from the article, although she made substantial contributions to the analysis and interpretation of data. This author and her contact details have now been added to the published article.
Also, the name of the tenth author, Adhemar Longatto, has been amended to Adhemar Longatto-Filho".



