Introduction
Neonatal pneumothorax may be spontaneous or secondary to pulmonary trauma and was first described in up to 4% of live births1. Modern estimates range from 0.05% to 6.7%, with rates at the high end of the range typically reported in very preterm and low birthweight babies, and babies at term gestation2-5. Little data is available outside of neonatal intensive care units (NICUs), especially amongst regional health services with paediatric facilities. Australian data is also sparse, but amongst special care nurseries capable of caring for infants born from 32 weeks gestation the prevalence was estimated at approximately 2% in neonates requiring oxygen therapy6. Numerous risk factors for air leak have been identified including prematurity, low birth weight (LBW), respiratory distress syndrome, congenital lung disease, respiratory insult including meconium aspiration syndrome (MAS), prolonged rupture of membranes (PROM), caesarean section and the use of mechanical ventilation3,7-12. Patient disease burden may include invasive ventilation requirement, prolonged admission and increased morbidity and mortality. Mortality among neonates with pneumothorax varies significantly, ranging from 12.5% to 43%, and is highest in preterm infants, infants requiring NICU support and in tension pneumothorax4,7,11-13. Treatment includes conservative management (oxygenation and nitrogen washout), invasive ventilation, invasive management (thoracocentesis or chest tube insertion) and surfactant therapy14. Oxygen therapy alone has been described as sufficient for the majority of term/late preterm infants, and room air has also been used15. Bilateral pneumothorax is less defined in the literature but is associated with cystic fibrosis transmembrane receptor gene mutations and increased need for ventilation13,16. Outcomes specific to bilateral pneumothorax have not been well described.
There is a lack of data reflecting the trend of neonatal pneumothorax in regional Australia. The aim of this study is to review the incidence and characteristics of neonates diagnosed with pneumothorax in Central Queensland, analyse outcomes in terms of the ability of local hospitals to manage this condition, and describe predictors for severe disease requiring transfer to a tertiary centre. Thus the role of regional health services in managing this condition will be reviewed.
Methods
Study setting
The Central Queensland region had a population of over 220 000 people in an area of nearly 120 000 km2 in 201517. A total of 5.7% of the populace identifies as Aboriginal or Torres Strait Islander. The region is serviced by three major regional public hospitals, designated hospitals 1, 2 and 3. Maternity services and birth registration included in the study were hospital based, while private services were not assessed. Hospital 1 is staffed by generalist paediatricians and possesses a special care nursery. Hospitals 2 and 3 are attended by rural generalist physicians (accredited by the Australian College of Rural and Remote Medicine) with paediatric qualifications, who are able to consult with on-call paediatricians at Hospital 1. Deliveries at less than 32 weeks gestation and neonates in need of intensive care are transferred to tertiary centres with NICUs. Such transfers involve significant logistical and clinical burden, with the nearest NICU being 600 km away by road and airlift.
Data collection
This was a retrospective observational cohort study, with data collected from medical records in the three regional hospitals in Central Queensland, Australia. Data were retrospectively collected for the period between 1 January 2008 and 31 December 2015. All births were registered by hospital medical records and patient selection was based on coding to ICD-10-AM (International Statistical Classification Australian Modification) category P25, ‘Pneumothorax originating in the perinatal period’. Exclusion criteria were lack of clinical or radiological pneumothorax diagnosis in the patient record, infants born at gestational age before 24 weeks or after 42 completed weeks, and age of more than 28 days at diagnosis. Standardised collection forms were used at all hospitals. Data collected included patient characteristics, risk factors and outcomes (Fig1). MAS included any meconium stained liquor at birth associated with respiratory symptoms, PROM as more than 18 hours between rupture of membranes and delivery, and LBW as birth weight less than 2500 g. Prematurity/preterm delivery was defined as gestational age less than 37 completed weeks by birth. PPV was defined as any form of oxygenation with pressure ventilation, including continuous positive airway pressure, biphasic positive airway pressure and bag-valve mask ventilation.
Population data in Central Queensland (number of births, gender of neonates, gestation at birth) was obtained from the same hospitals over the same time period, from summarised electronic hospital records data.
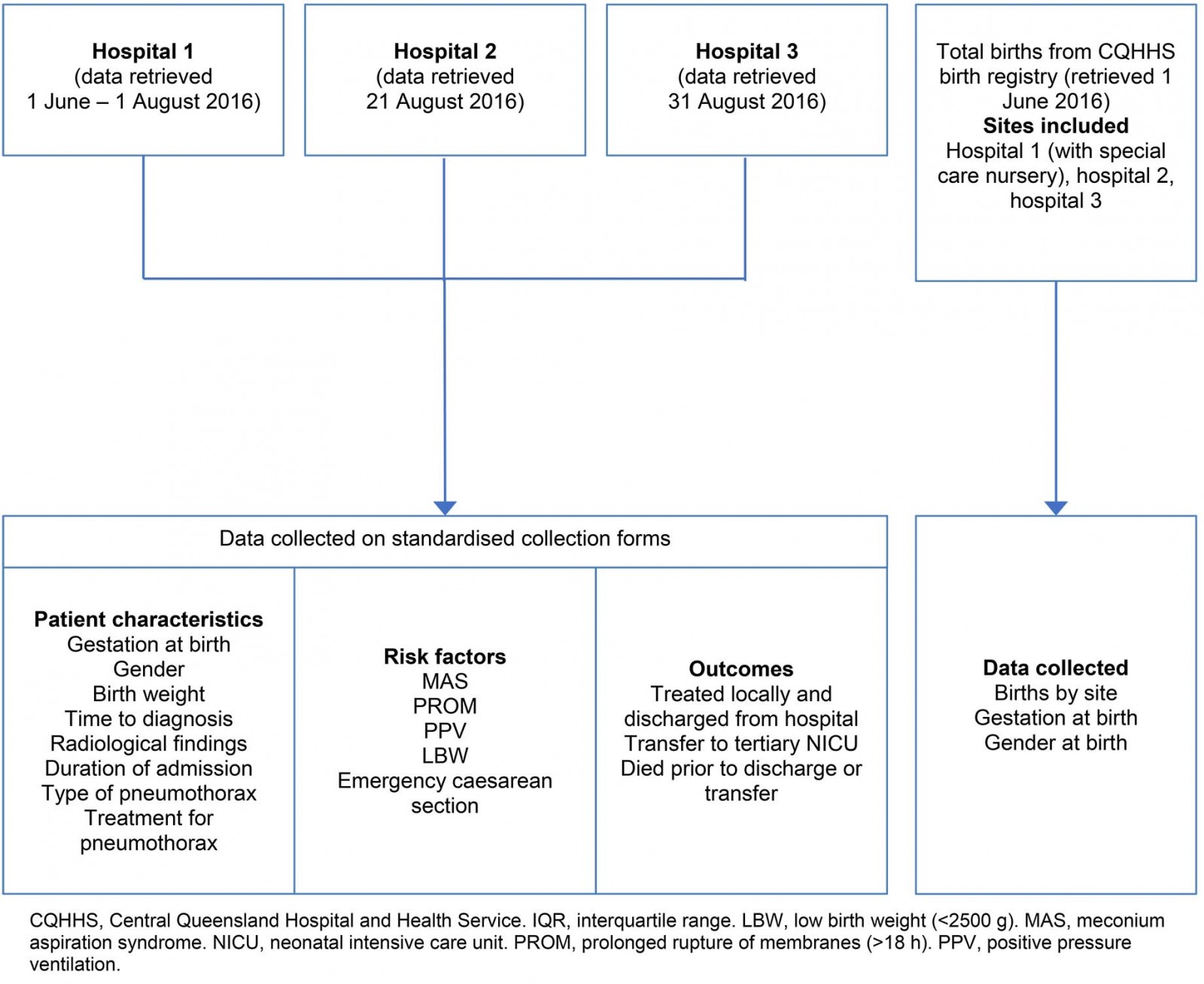 Figure 1: Types of data collected from study hospitals 1–3.
Figure 1: Types of data collected from study hospitals 1–3.
Measures of outcomes: Outcomes were separated into two categories. ‘Treated locally and improved’ refers to treatment within Central Queensland with cure of the patient and discharge into the community. ‘Transfer to tertiary NICU’ refers to a clinician-dependent decision during admission to escalate care to a tertiary centre. Patients who died before transfer were grouped together with ‘transfer to tertiary NICU’. Outcomes were followed up only until discharge from hospital, death prior to discharge or until transfer to tertiary NICU. Outcomes of transferred patients are outside the scope of this study.
Data analysis
All data were de-identified and analysed using the Statistical Package for the Social Sciences v22 (IBM; https://www.ibm.com/analytics/spss-statistics-software). Frequency statistics were performed for non-continuous variables. Distribution of data including means, standard deviation (SD), medians and interquartile range (IQR) were calculated for continuous variables. Pearson χ2 and Fisher exact analysis for categorical variables were used to test independence and significance. Student’s t-test and Mann–Whitney U-test were used for normally distributed and skewed continuous variables, respectively. Statistical significance was defined as p≤0.05.
Ethics approval
The use of data for this study was approved by the Human Research Ethics Committee of the Central Queensland Hospital and Health Services (HREC/16/QCQ/10) and the University of Queensland Ethics Committee (2016000900).
Results
Epidemiology
There were a total of 17 640 births in the three hospitals in the study period (Table 1). A total of 39 neonates were coded with a diagnosis of pneumothorax, with a total of 31 eligible patients enrolled in the study. The eight exclusions were incorrect coding, diagnosis outside of neonatal period or at uncertain age. The incidence of neonatal pneumothorax in Central Queensland was approximately 0.18%. Hospital 1 had the greatest proportion of cases of pneumothorax.
Patient characteristics
Gestational age ranged from 28.1 to 41.4 weeks and was skewed towards term gestation in both the pneumothorax cohort (median=39.6, IQR=3.7) and the Central Queensland population (median=39, IQR=2). Eight (25.8%) of pneumothorax infants were preterm. There were significantly more males than females with pneumothorax compared to all births at the same hospitals (Table 1). Mean birth weight was 3169 g (SD=744 g) with 6 (19.4%) classified as LBW (<2500 g) and 1 (3.2%) <2000 g. The majority of cases were admitted to the ward for treatment aged 1 day and diagnosed with pneumothorax within the first 48 hours of life. The most common admission diagnosis was respiratory distress, defined as any increased work of breathing including intercostal/subcostal recession, tracheal tugging, nasal flaring and tachypnoea. Other singular admission diagnoses include pulmonary hypertension, pleural effusion, poor feeding and sepsis. The median duration of admission at the delivering hospital was 5 days (IQR=8), with a range of 1–22 days. Ten infants (32.2%) were diagnosed with pneumothorax clinically without radiology recorded. Two-thirds of pneumothoraces were unilateral. At birth, 16 infants (51.6%) were actively resuscitated. Apgar scoring was performed in 30 infants – median 1 minute was 6.5 (IQR=4) and median 5 minute 8.5 (IQR=2).
Table 1: Distribution of cases and patient characteristics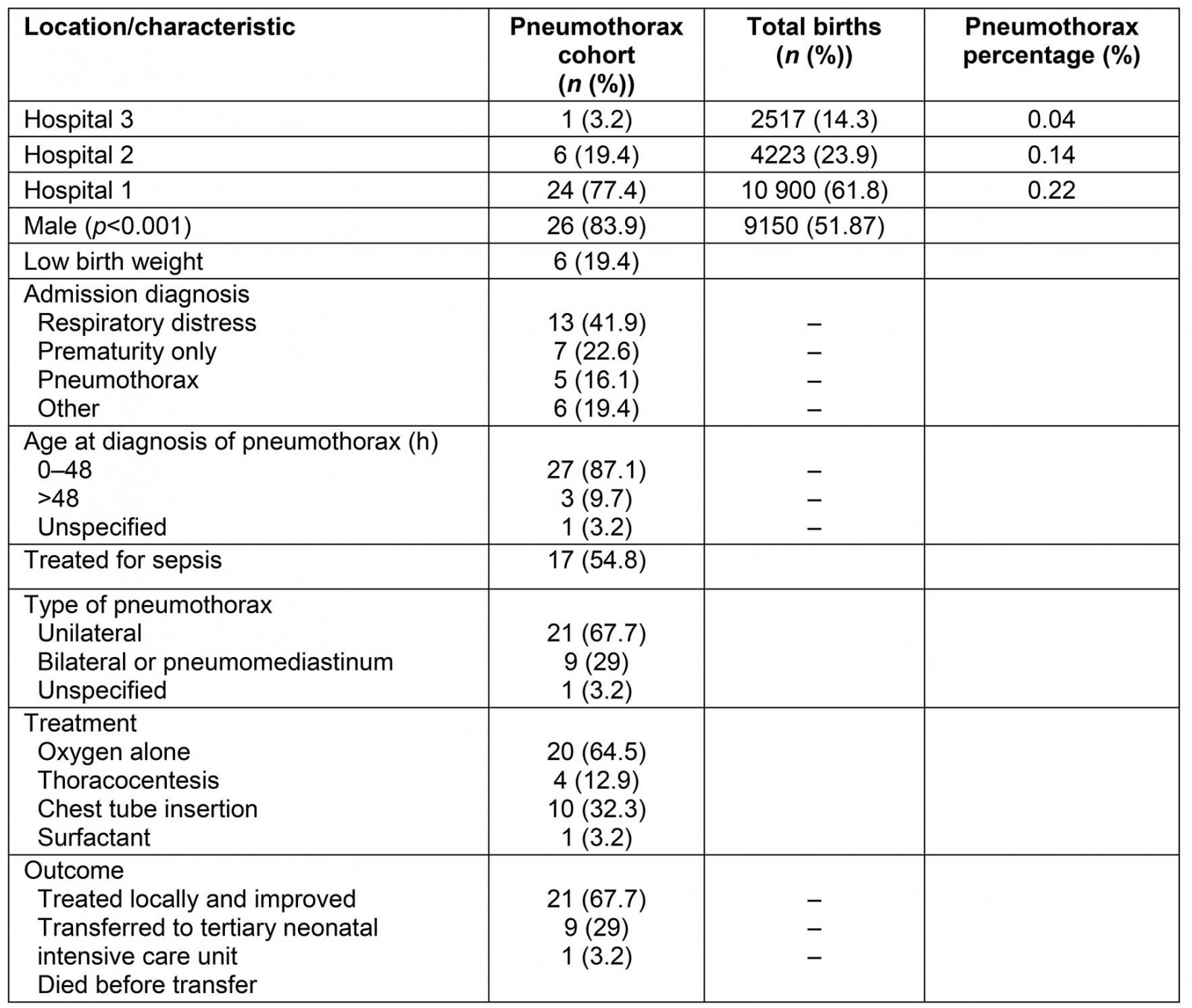
Risk factors for pneumothorax
Twenty one (67.7%) infants were exposed to continuous positive airway pressure, intermittent positive-pressure ventilation or bag valve mask resuscitation. Oxygen washout was the most common treatment, although up to a third of infants required chest tube insertion. MAS was noted in nine patients (29%) and PROM over 18 hours in four patients (12.9%), with one patient diagnosed with both conditions. Perinatal pyrexia was noted in a single patient. There were 14 (45.2%) caesarean section deliveries: 12 (38.7%) emergency and 2 (6.5%) elective. No statistically significant relationships were found between perinatal events MAS and PROM, LBW, sex, PPV or caesarean section with type of pneumothorax (Table 2).
Table 2: Patient characteristics and type of pneumothorax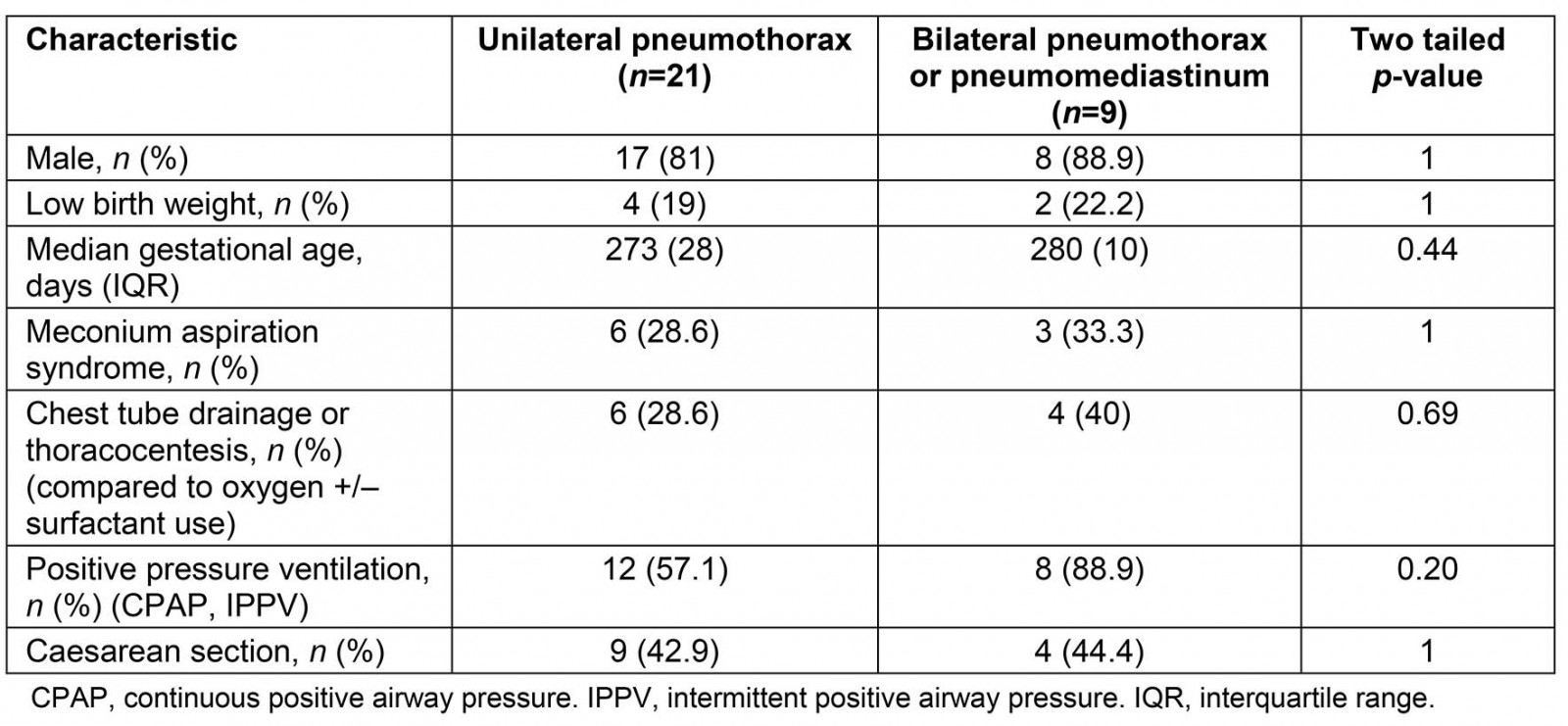
Outcomes for pneumothorax
Two-thirds of infants were treated locally, showed clinical resolution of pneumothorax and were discharged into the community, while nine were transferred to a tertiary NICU for further treatment. Need for transfer to tertiary NICU was significantly associated with bilateral pneumothorax (or pneumomediastinum) and need for chest tube insertion, but not with caesarean section, LBW, sepsis, gestational age or PPV (Table 3). One death was recorded for an infant born at 28 weeks gestation with concurrent limb hypoplasia, lung dysplasia and polycystic kidneys.
Table 3: Outcomes of neonatal pneumothorax in Central Queensland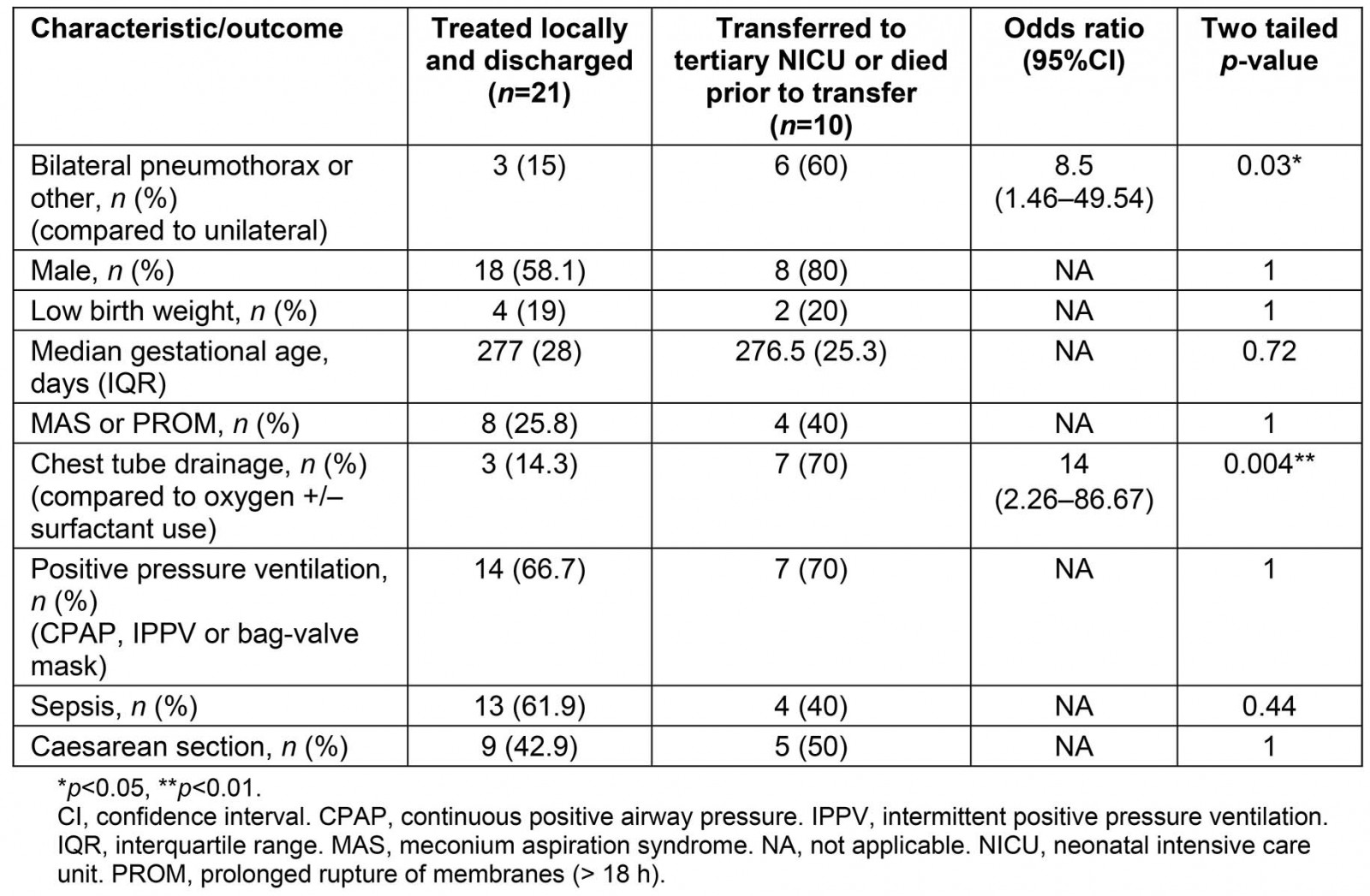
Discussion
The majority of cases were successfully managed in Central Queensland’s three regional hospital services. Paediatric units staffed by general paediatricians were able to either facilitate local management or transfer to tertiary centres based on clinical need.
The incidence of 0.18% was lower than rates of pulmonary air leak amongst neonates requiring respiratory support admitted to NICUs in Australia (4.2%)18 and rates of pneumothorax in preterm and term infants (1.3–9.2% and 6.7%)4,7,9. However, it was similar to studies in Denmark, NICUs in Bosnia, NICUs in India without gestational age restriction, and Canadian NICUs for term infants (0.13–0.35%)11-13,15. The higher incidences reported in the literature may be secondary to concentration of high risk/polymorbid infants. Similarly, Hospital 1 (the regional hub with a special care nursery) reported more cases compared to hospitals 2 and 3. The incidence of pneumothorax was higher (2%) across nine Australian special care nurseries of similar capacity between 2015 and 20176 than in the Central Queensland region, likely secondary to a greater proportion of preterm (>18%) and LBW infants (mean birth weight <3 kg). Median gestational age was term and similar for the pneumothorax cohort and Central Queensland population data collected. There was a greater proportion of preterm infants in the pneumothorax cohort compared to the Central Queensland population (25.8% v 6.9%). Prematurity and LBW were also more prevalent in pneumothorax infants (19.4% v 6.7% and 25.8% v 8.7%, respectively) compared to national surveillance data in Australia in 201719. This reflects existing literature regarding a link between prematurity, LBW and pneumothorax. Rates were, however, considerably lower compared to previous literature (48–83% and 50–60%, respectively), likely due to a concentration of NICU data in the literature12,13,20-22.
There were more males in the pneumothorax group than in the general Central Queensland cohort7-9,12,21,22. A definitive mechanism for this observation remains unknown. Gender was unable to predict type of pneumothorax and thus had reduced clinical utility in predicting prognosis. MAS was identified in nearly a third of the pneumothoraces, although this could not be compared to the Central Queensland population. Reported rates range from 9% to 24%4,8,22,23, and 30% in mechanically ventilated infants10. The high rates of MAS may reflect non-specific selection criteria for infants or an unknown external influencer.
The most common presenting complaint on admission in neonates with pneumothorax was signs of respiratory distress, which is consistent with known literature21. Interestingly, over half of cases were treated empirically for sepsis, which can present with similar signs of respiratory distress. Rates of PROM and maternal pyrexia were lower than sepsis, and it is possible that some cases of presumed sepsis were actually pneumothoraces. The lack of microbiological confirmation of infective agents in any sepsis case supports this possibility.
Bilateral pneumothorax or pneumomediastinum was a risk factor for transfer to tertiary NICU (p=0.03). Increased mortality and morbidity in bilateral pneumothorax have previously been observed23. Risk factors for bilateral pneumothorax could not be identified in this study. Although caesarean section has previously been linked to pneumothorax through increased use of continuous positive airway pressure9, neither appears to predispose to bilateral pneumothorax. Similarly, respiratory insult from MAS bears little correlation to type of pneumothorax. Unfortunately, no infants were tested for cystic fibrosis in this study. It is possible that a separate, unidentified mechanism of respiratory injury or underlying disease is responsible for bilateral pneumothorax. The predisposition for chest tube insertion in neonates transferred to a NICU setting is likely an observation of increased disease severity. However, there was no statistically significant link between use of chest tube or thoracocentesis and type of pneumothorax (p=0.53). Thus it appears a significant proportion of patients with unilateral pneumothorax also required chest tube insertion. As such, while bilateral pneumothorax/pneumomediastinum is a predictor of poorer outcome, it does not predict selection of management modality.
A majority of patients were exposed to positive pressure ventilation. Similarly to chest tube requirement, use of such ventilation was not strongly related to type of pneumothorax (p=0.69). While positive pressure ventilation, in particular continuous positive airway pressure24, has been linked to pneumothorax, the lack of association with type of pneumothorax limits its utility in predicting prognosis in this study.
Two-thirds of pneumothorax patients had good clinical outcomes. The single reported mortality was delivered at less than 28 weeks gestation and had several congenital abnormalities incompatible with life. Thus it is difficult to attribute mortality as an outcome of pneumothorax. An observed all-cause mortality of 3.2% is limited because it does not include mortality following NICU transfer. Aly et al reported a similar 3% mortality for non-LBW infants21, although total mortality among cases was 15% and ranged from 13% to 36% in prior descriptive studies, despite a lower proportion of bilateral pneumothoraces8,11,13,20,22. Amongst very preterm infants less than 28 weeks gestation, mortality has been estimated at 43%7 and at 23.5% in neonates less than 1500 g3. In the Central Queensland cohort, LBW and emergency caesarean section were not risk factors for poorer neonatal outcome (p=1). This is in contrast to prior affirmations of increased duration of hospitalisation and mortality in LBW infants with pneumothorax7,20,21. It is likely that the relative scarcity of very LBW (<1500 g) neonates (one patient, the aforementioned mortality) is responsible for the lack of association in the data. Overall length of stay in hospital was also reduced compared to the literature (2–38 days)11,13, although lower proportions of underweight and preterm infants are described in this cohort. Age of diagnosis was also comparative to studies in regional hospitals and tertiary NICUs12,13. The ability to diagnose the majority of pneumothoraces within 48 hours of delivery likely contributed to the low mortality rate.
This retrospective study has some limitations. Only sex and gestation data were available from the Central Queensland births between 2008 and 2015 data for comparison. Further data would have allowed better comparison regarding the relative distribution of perinatal events, birth weight, delivery method and outcome. As such, the influence of these factors on the profile of pneumothorax in neonates and clinical outcomes was not assessed. Ventilator parameters in the neonatal period were omitted from this study due to data inconsistencies, and thus limited the analysis of ventilation and pneumothorax. As discussed above, end outcomes for patients transferred to tertiary NICU centres were outside the scope of this study, which could confound measures of outcome and artificially ameliorate disease severity.
Conclusion
This study provides an estimate of incidence over an 8-year period of neonatal pneumothorax in Central Queensland that is similar to that of other descriptive studies. Risk factors for pneumothorax were similar to those identified in the literature. Bilateral pneumothorax was found to be predictive of poorer outcome, but no risk factors were identified. Importantly, more than two-thirds of cases were successfully managed locally. A single recorded mortality existed that could not be attributed to limitations in regional health care, suggesting all patients were treated appropriately or transferred to a tertiary unit when indicated. The excellent outcomes may be secondary to the low proportion of LBW and preterm neonates with pneumothorax in the Central Queensland region. This observation highlights the capability of regional hospitals and general paediatricians in treating this condition. Future research should aim to clarify local population characteristics in comparison to pneumothorax cases, risk-stratify subpopulation groups in the rural community and explore longer term outcomes including those of transferred infants.
