Introduction
Immunization is one of the most cost-effective interventions for reducing mortality and morbidity, as well as disability, in infants1,2. The implementation of routine vaccination in infants has resulted in decreasing vaccine-preventable diseases3,4. WHO has issued a policy on the Expanded Program for Immunization to prevent the population from acquiring six diseases, namely tuberculosis, diphtheria, tetanus, pertussis, measles, and poliomyelitis4,5. WHO has set the goal of vaccination in a global immunization vision and strategy6. WHO articulated that every country should achieve the goal of 90% of children aged less than 1 year having immunization coverage nationwide and at least 80% in every district/municipality in the country by the year 20206,7. However, immunization coverage above 90% has still not been achieved and is a problem in some countries that have very little resources8-10. With a population of more than 257 million people, Indonesia has more than 5 million children to be vaccinated every year11,12. Although the vaccination coverage in Indonesia is increasing every year, it is still far from WHO’s goal commitment7. Indonesia’s population and health survey shows that, in 2012, only half (53.6%) of Indonesian children had received complete basic vaccination11; in 2017, the proportion of Indonesian children who had received complete basic vaccination increased slightly to 58.22%12. Considering the Indonesia Health Profile in 2016, from 34 provinces in Indonesia, only 12 provinces have achieved national vaccination targets set by the government13.
Previous literature has demonstrated that the characteristics of children, parents, and households are an important predictor of child immunization14-16. As to some characteristics, there is conflicting evidence of the gender gap in immunization, which may reflect specific cultural differences in status. This finding revealed that boys are more fortunate than girls in India17. Other studies have found the opposite in Nigeria, but this gender difference is not found in Swaziland18 and Togo19. Moreover, the variables associated with uptake of immunization are parity, child order20,21, age of baby, and place of birth22,23.
For parental-specific characteristics, educational attainment and literacy6,7,20,23, employment status22,24,25, age and age at birth23,26, marital status19,27, and religion19,23 have strong relationships with the vaccination status of children. These characteristics have great relevance to the extent and quality of health care mothers are able to provide to their children26. Household characteristics are influential in their effect on children’s health outcomes, which are related to economic and social status as measured by wealth or property. The impact of these features on the immunization coverage of children is unclear after studying the reflections of national differences. For example, Bugvi et al22, Olorunsaiye and Degge23, and Singh and Parasuraman28 reported inequality in the vaccination of children in the absence of parents in the family and for children living in households with lower socioeconomic status. In contrast, Babirye et al29 found that the vaccination rate decreased in children of high socioeconomic status. However, the overall results of these studies indicate that there is a variety of factors related to costs and time for mothers to be able to access health facilities to vaccinate their children28,30.
Moreover, a number of studies report disparities in immunization levels based on residential areas in developing countries. Previous studies reported inequality of rural children in receiving vaccines in Ethiopia31, Nigeria21, and Pakistan22. A study in India showed that vaccination rates have decreased among urban children, especially in slums and informal settlements in this area32. These differences may be due to differences in development strategies on immunization coverage.
The difference in the ability to access health facilities between villages and cities is also an important factor, where access in rural areas is usually lower than in cities. Previous research by Ibnouf et al33 and Rup et al34 revealed that distance to healthcare providers is an important factor in accessing immunization treatment.
The phenomenon of the incompleteness of immunization in Indonesia still needs to be investigated so that the relevant factors and causes will be known. However, from past literature reviews15,18,19,23, studies are limited because they only consider household-level factors. The characteristics of the residential area play a very important role in the success of the immunization program in Indonesia because the regions of the country have different geographic and economic characteristics24. In addition, most of the previous studies were cross-sectional studies and used only one level of logistic regression analysis23-27, which is not enough to predict behavior affected by hierarchical structures at the community level.
This study implements the theoretical model of healthcare utilization by Andersen. The model consists of three main factors, namely predisposing, enabling and reinforcing characteristics35. Predisposing factors are the personal preference for using services based on demographic, religious, and health-related values and illnesses. This study included predisposing factors, enabling variable and primary health center, while the dependent variable is the completeness of the basic immunizations recommended by the government. The purpose of this study was to identify a three-level model, including child level, parent level, and community level to analyze determinants of child immunization status in Indonesia based on the Indonesia Demographic and Health Survey 2017 (IDHS 2017).
Methods
This research drew upon data from IDHS 2017, which was accessed through the Demographic and Health Surveys (DHS) Program. The DHS is an important series of surveys that provides important information about health conditions, nutrition, and demographic indicators. The IDHS 2017 successfully interviewed 49250 eligible women aged 15–49 years. Multistage random sampling was used to obtain representative samples from all provinces in Indonesia. In the 2010 population census of Indonesia, there were 1970 census blocks for used as the determination of the first sampling stage, then 34 census blocks representing the number of provinces in Indonesia were selected. In the second stage, eight families were randomly selected for each selected census block. For this study, data were selected pertaining to babies, living with mothers, aged 12–24 months. Accordingly, the sample encompassed 4753 children.
For the purposes of analysis, children under 1 year were not included because they are not old enough to receive all basic vaccination schedules in Indonesia. The dependent variable in this study was the immunization status of children. The immunization status of these children was further grouped into two groups: if a child was completed immunized, it will be coded as 1; otherwise, it was coded 0. Immunization was said to be complete if the baby or child had received basic immunization consisting of the bacille Calmette-Guérin (BCG), diphtheria, tetanus, pertussis (DTP), polio, measles, and hepatitis B vaccines. This routine immunization is scheduled to be received by children up to the age of 12 months12. The sources of information on the immunization status of children were obtained from the Buku Kesehatan ibu dan anak (maternal and child health book) or Kartu Menuju Sehat (child health card), owned by the mother and child. This book/card is a record of the health status of mothers and children, including immunizations received by both mother and child. In Indonesia, the ownership of this book/card is higher than 87%. Accordingly, the immunization data based on these records were a reliable reference for a survey. Where there was no maternal health book or child card, and the mother was uncertain about the status of the child, the child’s immunization status was categorized as incomplete36.
The independent variable was chosen based on a theoretical study and a literature review that has been published. The conceptual framework used as the basis for developing the model in this study was Andersen’s behavioral health model35. The children’s sex was grouped into male and female. The age of children was counted in months. The birth order was defined as the list of birth from the order of birth in the child’s family. The mother’s age when giving birth (15–49 years) was categorized into groups at 5-year intervals. The educational attainment of mother and father was ‘no education’, ‘primary level’, ‘secondary level’, and ‘higher level’. Mother occupation status was ‘do not work’ and ‘working’. Women’s wealth status refers to the five quintile groups of household wealth36. The household wealth index was developed by totaling household assets and amenities (radio, television, refrigerator, bicycle, motorcycle or car) and housing characteristics (electricity, flooring, wall/roofing, water source, latrine ownership and bedroom)36. It was categorized into quintiles from lowest to highest. The antenatal care (ANC) was ‘received ANC’, and ‘no ANC’; however, for the frequency distribution, the ANC was described as the number of maternal visits to health facility.
The residential area was grouped into urban and rural areas. The proportion of puskesmas (public health centers) was the number of puskesmas compared to the population in each province, then categorized into quintiles from lowest to highest36,37.
STATA version 14 (https://www.stata.com) application was used to analyze the data. Frequency distribution was applied to describe the respondents’ characteristics. Cross-tabulation was employed to demonstrate the proportion of different categories with respect to immunization status. Multilevel logistic regression was implemented to estimate immunization status in a multivariate context. Model fitting using residuals was checked. Three model levels were categorized as child level, parent level, and community level. The child level included child’s sex, age, and birth order. The parent’s level included mother’s age at delivery, mother’s education, father’s education, father’s working status, ANC status, residence, and region. The provincial proportion of puskesmas was included as the third level. The data were run using xtmelogit in Stata, household wealth was introduced as random factors in the multilevel analysis38,39. The statistical results were expressed as adjusted odds ratio (OR) with t-statistic. The hypothesis was rejected at p-value greater than 0.05.
Ethics approval
This study has been approved as ethical research by The Institutional Research Board of the Faculty of Public Health, Diponegoro University, Indonesia (25/EA/KEPK-FKM/2020). Approval for the use of IDHS 2017 data was granted by the Measure Demographic and Health Surveys (DHS) Program.
Results
Descriptive statistic
Distribution of the study participants across the sociodemographic variables is presented in Table 1. Gender numbers of children were similar: 51.67% male and 48.33% female. Most (92.7%) were the first-born child. Most (52.09%) of the mothers were aged between 25 and 34 years. Of the mothers, 28.30% had vocation-level education; of the fathers, 31.41% had senior high school-level education. Almost half (49.55%) of mothers did not work outside the house, and the largest occupational group, fathers (26.76%), were agricultural workers. Among the participants, 54.30% were from rural areas and 32.29% were from the Java and Bali region. Of the mothers, 81.46% had completed an ANC visit. Regarding economics, most (51.72%) of the children’s families were of the most impoverished wealth level. Most of the children’s families were located near a centralized health center, with an integrated healthcare center (43.32%), village health service center (40.31%), policlinic (private clinic run by medical practitioner) (42.94%) and public health center (42.94%) at the impoverished levels.
Immunization coverage rate among study participants
The immunization coverage across the sociodemographic variables is presented in Table 1. The IDHS dataset contained information for 4753 children whose mothers completed the questionnaire for married women. The proportion of full immunization was 58.22% (2767 out of 4753 children). Full immunization coverage was similar across sex (57.49% for male and 58.99% for female). In this study, first-born babies were less likely to have full immunization (57.19%) than second-born babies (70.97%). Concerning maternal age at delivery, the completed vaccination coverage rate was 51.12% for 15–24 years, 59.53% for 25–34 years and 61.46% for 35 years or older. Regarding education level, full immunization was highest among children whose mother graduated from vocational education (63.35%) and whose father graduated from vocational education (63.16%). Mother’s occupation seems to be important. Mothers who had no occupation were less likely to have full immunization (45.99%) than mothers who have an occupation. Furthermore, full immunization was highest when fathers worked as managers and in administration (70.41%) followed by professional/technical occupation (66.50%). Moreover, complete immunization was higher among those who completed ANC (59.74%) than those who did not compete ANC (51.53%). The rate of complete immunization coverage was 55.33% in rural area settings, which was lower than in urban settings (61.65%). In terms of household wealth, incomplete immunization coverage was found to increase with the declining economic status of children’s families (34.83% for the highest quintile to 47.98% for the lowest quintile). Importantly, for complete immunization there were disparities among regions.
Considering health facilities coverage as presented at Table 1. It was found that there was no clear relationship between immunization coverage and the proportion of posyandu (integrated health centers), poskesdes (village health services), and policlinics. Children living in areas with the lowest number of public health centers tend to receive less complete vaccination coverage than children in areas with those health services in the highest proportion.
However, in the multilevel analysis, the model did not include the proportion of posyandu, poskesdes and private policlinic (policlinic) due to they have multicollinearity problem. Accordingly, for the community level variable, the multilevel analysis only applied the proportion of puskesmas.
Multilevel logistic regression analysis
The child level included sex of child and birth order. After adjusting for wealth in Table 2, it was found that children of second birth order (OR=1.934) had a 93.4% higher likelihood of receiving complete immunization than the first child.
The parent level included sex of child, birth order, age of mother at delivery, mother and father’s education, mother and father’s occupation, antenatal care visit, residence and region. The education of the father and the mother’s occupation were removed from the model due to collinearity problems. After adjusting for household wealth, children of mothers who were older at delivery had a 3.4% (OR=1.034) higher likelihood of receiving full immunization than children of mothers who were younger at delivery. Regarding birth order, first borns were less likely to be vaccinated than second order or higher births. Children of mothers with university (OR=3.347), vocation (OR=3.731), senior high school (OR=3.419), junior high school (OR=2.131) and primary (1.809) education level had an increased likelihood of receiving full immunization compared to children of mothers with no education.
After taking household wealth level into account, the likelihood of receiving full immunization among mothers who had completed ANC (OR=1.199) was 19.9% higher than among mothers who did not complete ANC. Regarding the region, children of mothers from Java and Bali (OR=2.080), Nusa Tenggara (OR=3.298), Kalimantan (OR=1.584), Sulawesi (OR=1.879), and Maluku (OR=1.680) regions had increased likelihood of receiving full immunization compared with children of mothers from the Sumatra region.
In the community level model, the proportion of PHCs (highest, high, medium, low, lowest) was added into the model. The result showed that children whose mothers were resident in communities with highest (OR=1.290), medium (OR=1.316) and low (OR=1.440) proportion of PHCs had a higher likelihood of receiving full immunization compared to children of mothers residing in communities with the proportion of PHCs at the lowest level but the ‘high’ proportion of PHCs did not significantly influence full immunization.
Table 1: Immunization coverage across the sociodemographic variables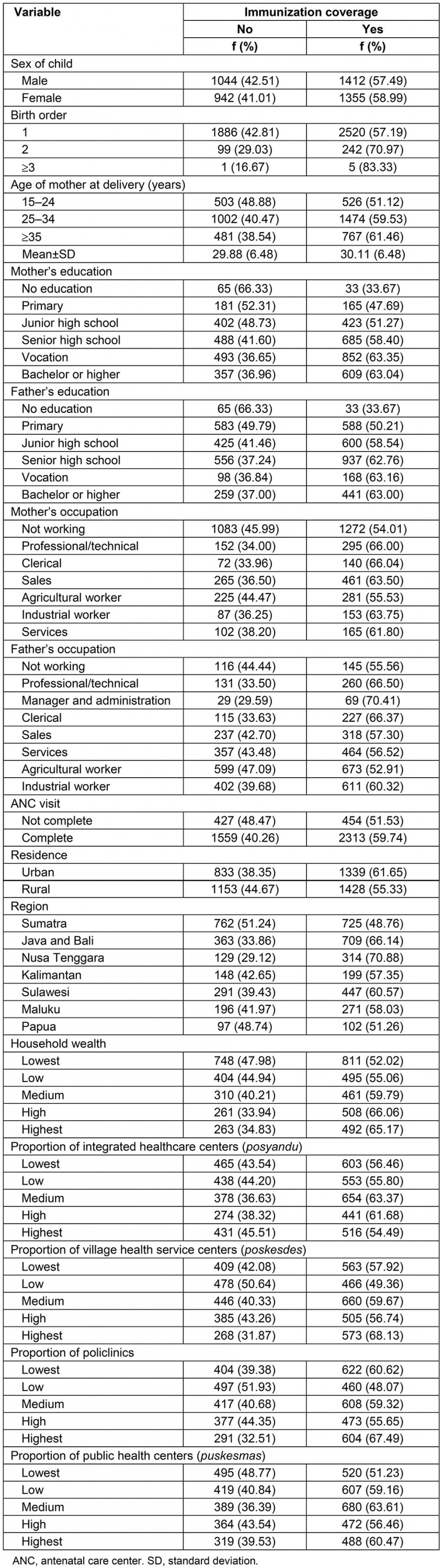
Table 2: Multilevel logistic regression analysis of immunization coverage among children aged 12–24 months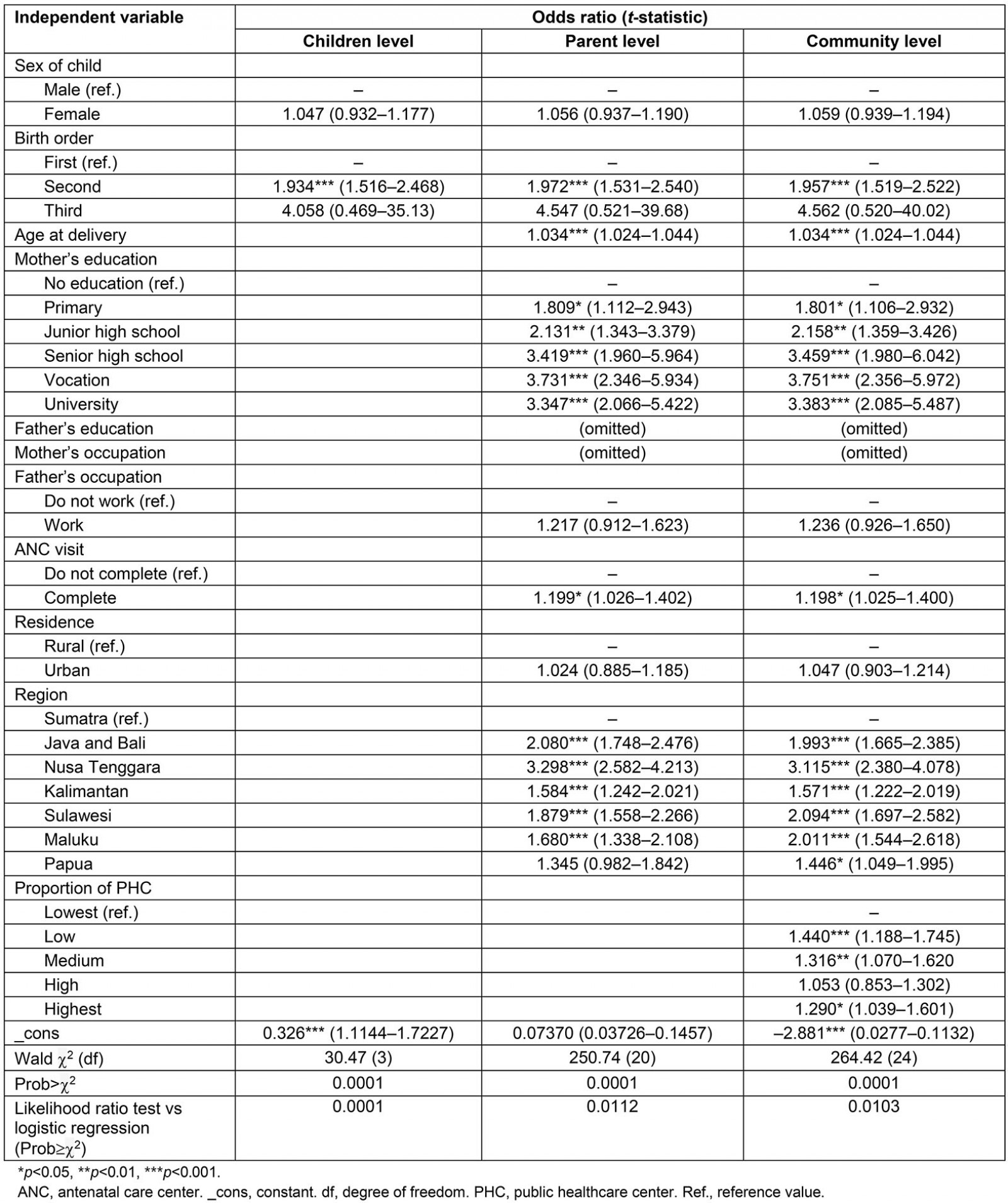
Discussion
Using representative national data from Indonesia, it was found that 58.22% of children aged 12–24 months were fully vaccinated. However, the percentages found in this study are far from the WHO and UNICEF’s global immunization goals, vision and strategies, which outline a goal of 80% coverage7. Therefore, finding out how socioeconomic factors and community characteristics affect the status of child immunization is crucial to improving vaccination coverage in Indonesia.
Vaccination coverage in Indonesia appears weakly associated with region after accounting for all confounders. Although birth order, age of mother at delivery, mother’s education, father’s occupation, ANC and proportion of PHC were strongly associated with vaccination coverage, the marginally significant result obtained for the region does not mean that the influence of location on vaccine uptake should be excluded. On a regional basis, it was found that geographical differences and the size of the population were significantly related to achieving complete immunization coverage40,41. The highest coverage of complete immunization was found in the Java and Bali region, whereas the lowest complete immunization coverage was in the Sumatra region. In Indonesia, geographical conditions make some areas isolated or remote, and other areas are difficult to reach because of the limited availability of roads and public transport40,41. Low immunization coverage is often found in distant, rural areas, and where there are religious restrictions and fear of side effects from vaccines41. Some research has found that certain regions have their own beliefs about health and that it is a challenge for health professionals to try to look after mothers’ health42,43. This is also caused by a lack of knowledge and awareness about child immunization44,45. For this reason, in this difficult area, an innovative approach is needed to achieve immunization targets. The needs of supply and demand must be met. For example, outreach programs with small mobile units have great potential to reach remote areas in the mountains. The use of local transportation to reach remote areas in Bangladesh has been highly recommended44. This approach is very appropriate for reaching children far from health facilities44-48 so that the overall, low coverage of immunization in children can be addressed and the numbers improved49.
As birth order increased, the likelihood of the child being vaccinated increased50,51.This finding contradicts other findings that an experience of immunization side effects in vaccinated babies might have triggered parents’ negative perceptions about vaccination52. If a first child experiences side effects from vaccination, parents may be less likely to vaccinate their second child. Therefore, the chances of a second child being vaccinated are reduced 53. Moreover, it was found that older mothers are more likely to complete the vaccination of children51,52; children of older mothers are more likely to be fully immunized than children of younger mothers, who are often unable to make their own decisions. Younger mothers have to discuss the decision with family members. Older mothers are more likely to have parenting experience and are more likely to have knowledge of the child’s health in general54. The importance of maternal education in child health is internationally recognized. Children with highly educated mothers are more likely to be fully vaccinated51,54. Older mothers have possibly been exposed to more immunization information, and have experience in terms of access to information and better health services15,18,27. Knowledge and experience of access to health services is what drives the completeness of child immunization41,49. Also, highly educated mothers tend to have better knowledge and understanding of general health and the benefits of immunization50. As previous studies have shown, level of education is significantly related to the level of public health awareness51,52. This can be explained by those having better education having a better understanding of immunization recommendations46. The studies also found that children of mothers who routinely do health checks tend to have complete immunization compared to those who do not do health checks during pregnancy and postpartum; this is because mothers who do the checks receive more complete information or health education. Danchin et al’s research revealed that information about immunization was received by mothers while pregnant53. It was also found that maternal education affects whether mothers remember whether their children have been vaccinated, and whether the vaccination card is kept for the child50. This means that education improves mothers’ understanding of the importance of vaccination and child health care. Therefore, ensuring access to education for women in remote areas should reduce the disparity in vaccination54,55.
The completeness of immunization was confirmed as significantly correlated with ANC45,56; mothers who received more information about the benefits of vaccines tended to increase their access to vaccination services for their babies. So, it can be concluded that the ANC visit is not only beneficial to the health of the mother, but it encourages the mother to have a broad insight about health; for example, the benefits of vaccination57. A mother’s visit during a pregnancy check will increase her interaction with health workers, and this communication can increase the flow of information about health, in this case, the benefits of vaccination, reducing the fear of side effects58.
The findings provide very good input for public health worldwide. Public health status is determined not only by modern treatments, but also by effective, widespread public education. In addition, government stakeholders can be encouraged by the findings to increase community education and further increase the dissemination of health information to the wider community40,41. Furthermore, it is suggested that one way the Indonesian government could increase the coverage of universal child immunization would be by long-term investment in increasing parental education54.
The results of the study also show the importance of the quality of service received by mothers during their ANC and postpartum delivery. If the quality of service is good, health workers will provide the necessary health education and information about immunization51,54.
Considering the community level determinants, an increase in the number of public health centers was associated with higher vaccination coverage. Children whose mothers reside in the close vicinity of healthcare centers were strongly positively associated with immunization coverage. Previous research has shown that increasing the number of health centers in a village to 1 per 1000 people increases the likelihood of children being fully vaccinated 54%59. Similarly, several studies60-62 have found that complete immunization of children is related to the use and effectiveness of maternal health services. However, immunization coverage is still incomplete, particularly among children born at home63,64.
The findings, consistent with regional analyses and their relationship to vaccination coverage, underscore the need to bridge the gap in vaccination inequality in Indonesia. The public healthcare center (puskesmas) is a community health service facility in Indonesia under the Ministry of Health. Postpartum midwifery visits are important to the integrity of the immunization status. Indonesia’s midwifery recruitment program in villages across the country is relevant in this context, aiming to bring maternal and child health services to remote areas and disadvantaged communities63,64 even when those communities are hard to reach65.
Regulation of the Minister of Health of the Republic of Indonesia No. 43 of 2019 states that puskesmas play a role in fostering health cadres in running posyandu (integrated health centers)65,66. If a posyandu has active cadres, promotional and preventive health activities are also expected to run well. One of the important activities in posyandu is the immunization program67. Posyandu cadres have a role in encouraging mothers and parents to bring their children to the posyandu to get immunization67. Therefore, cooperation and posyandu guidance carried out by the puskesmas is very important. Moreover, the role of the village midwives may also be related to posyandu activity. Vaccination in a posyandu is mostly introduced and performed by village midwives67,68. Village midwives also have a role in outreach programs such as special vaccinations during home visits for maternal health. This outreach program may have accelerated the immunization coverage69.
This study also reveals that the number of healthcare facilities managed by the community is very meaningful in achieving complete immunization. Accordingly, it is recommended that the Indonesian government increase the availability and the number of health facilities70. Moreover, the ease of access to immunization in various health service facilities should also be prioritized.
From the results of this research and discussion, it is recommended that the government increase the number of health facilities, especially in areas that have low immunization coverage. Likewise, mothers’ knowledge about immunization needs to be improved so that they pay more attention to health during pregnancy and to immunization for their children. In addition, it is hoped that the government can design priority programs to improve the quality of health services and health education, especially for people with low education.
Conclusion
This study’s findings indicated that there is a wide range of inequality in immunization throughout Indonesia due to socioeconomic and demographic factors. Complete immunization status was significantly associated with birth order, age of mother at delivery, mother’s education, father’s occupation, ANC, region and proportion of public healthcare centers. This study emphasizes the need to increase the number of health centers in each community with the objective to narrow disparities in maternal and child health services. It is recommended that the Indonesian government provide guidelines for immunization and implement relevant policies, including monitoring and evaluation of immunization, especially for regions with low vaccination coverage levels. This conclusion is made in the context of strengthening the role of the community in the fight against diseases preventable by vaccines in developing countries, which is critical to the success of immunization and the general health of society.
Limitations
Although this study has articulated an important finding, it has some limitations. This study did not cover the time of giving vaccination, in which the time of giving vaccination is related to immunization effectiveness. In terms of data collection, this study ranges to 2 years before data collection, which can result in a bias recall on this study. Furthermore, due to the large area of Indonesia, the results of this study could not be directly implemented in developing health policy for particular areas.
Acknowledgements
The research team highly appreciates Rajmangala University of Technology Thanyaburi, Thailand, and the Faculty of Public Health, Diponegoro University, Indonesia, for all the support during the writing process. Furthermore, the team thanks all colleagues who provided suggestions and input towards the concept of this article. The researchers also thank the Measure DHS for granting permission to use data from Indonesia’s Demographic and Health Survey results.
References
You might also be interested in:
2010 - Investing in mother's education for better maternal and child health outcomes
2001 - The impact of health system reform on remote health in Cambodia and the Philippines

