Introduction
Parkinson disease (PD) is a complex neurodegenerative disease that requires ongoing and increasingly more complex health care as the disease progresses. Moreover, the required health care is specialized and requires a team of PD healthcare specialists to provide optimum care1-3. Bloem et al report that PD healthcare specialists are more likely to adhere to PD treatment guidelines than professionals with generic training and are more aware of the role that other healthcare specialists offer for people with PD3. This highlights the importance and necessity of specialist care for people with PD. Although the management of PD is optimized by a specialized team of healthcare providers, for most people with PD, even in large urban areas, their care is often fragmented with poor interdisciplinary collaboration and a lack of resources and access3.
For people with PD who live in rural areas, these problems are exacerbated because few PD specialists live and practice in rural areas. Work by Singh et al supports this assertation in a qualitative study on people with PD living in rural Wyoming4. The group found that people with PD have significant challenges in accessing information about PD, gaining access to PD healthcare specialists, and accessing community support for their condition4. Because people with PD in rural areas lack access, they often have a difficult choice to make by either traveling long distances to see PD specialists, choosing local generalists to manage their condition, or forgoing PD specialized care altogether. Clearly, these choices are not ideal when managing a complex and chronic condition like PD, and delays in access can prolong or exacerbate health-related problems, which in turn can lead to suboptimal care. Without ongoing, coordinated services from specialists, people with PD in rural areas are at a distinct disadvantage to their counterparts in large, urban cities, who have access to many specialists with expertise in PD. Therefore, increasing access for those in rural areas is important in alleviating the disease burden for those with PD and their caregivers.
One solution to the health disparities perpetuated by inadequate healthcare access for people with PD living in rural areas is telehealth. The growth of telehealth has been expedited by the recent pandemic5-9, but there is still a need to build evidence for the feasibility, safety, and efficacy of telehealth for people with PD10,11. Several researchers have reported aspects of feasibility and safety of telehealth for people with PD12-16. For those with PD who live in underresourced areas or are homebound due to their advancing PD, telehealth increases access to specialized care17. Bush et al reported that people with PD living in rural Wyoming were interested in pursuing advanced technology options to address the barriers to their health care18. Studies have also suggested that telehealth decreases the cost and travel burden for people with PD19-21. Additional, telehealth can be used as a tool to minimize patient exposure to contagious pathogens22 and this is especially important in vulnerable populations like those with PD. While these studies support a growing foundation of evidence, all of the aforementioned studies were centered on ‘telemedicine’ rather than on a more collaborative, multidisciplinary care plan involving other important members of the PD healthcare team as cited in Bloem et al3. People with PD also cite a lack of care coordination as a healthcare barrier for those in rural communities4. Therefore, there is a need to provide foundational evidence for a multidisciplinary healthcare team approach for telehealth for people with PD in rural communities. Moreover, this approach might support the Triple Aim Initiative by the Institute for Healthcare Improvement. They recommended that the US healthcare system may be improved by improving the patient care experience, improving population health, and reducing healthcare costs23.
This study aimed to provide evidence for the feasibility, safety, and signal of efficacy of a coordinated, multidisciplinary telehealth program consisting of evidence-based speech therapy24-26, physiotherapy27-31, and medication management/pharmaceutical care32-34. The first aim was to test the feasibility of this coordinated telehealth program over an 8-week period. For this aim, it was hypothesized that the withdrawal rate would be less than 12% and that 80% of participants would attend 80% of their schedule treatment sessions for each of the three treatments in the telehealth program over the 8-week study period35. The second aim was to assess the safety of the coordinated telehealth speech therapy, exercise therapy, and pharmaceutical care. For this aim, it was hypothesized that there would be no major adverse events, including falls, cardiac events, and injuries, over the course of the 8-week telehealth program. The third aim was to detect a signal of efficacy of the coordinated telehealth program across several domains of outcomes, including communication effectiveness, physical performance measures, medication adherence, and disease-specific mobility and quality-of-life measures. Identifying a signal of efficacy is important for determining the direction and design of future research on this telehealth program.
Methods
Design overview
This was a pilot, prospective cohort, phase II study on the implementation of a coordinated, 8-week telehealth program, consisting of speech therapy, physiotherapy, and pharmaceutical care, using a single cohort of people with PD living in rural Wyoming and Nevada, USA. Participants were assessed in the ‘on’ PD medication state before and after the 8-week telehealth program as well as a 6-month follow-up assessment. The study was coordinated by a PD nurse and also an assistant study coordinator who helped with scheduling and technology.
Participants
The inclusion criteria were the following: English speaking, diagnosed with idiopathic PD, taking at least one PD medication, Hoehn and Yahr stages 1–4, aged 30 years or older, commitment to attend scheduled sessions, access to internet to participate in telehealth sessions, and allowing communication to primary care provider. The exclusion criteria were the following: dementia or inability to follow directions; skilled nursing resident; comorbidities that would preclude exercise participation or increase participant risk (severe osteoarthritis/pain, stroke, severe respiratory problems, traumatic brain injury, neuromuscular disease, atrial fibrillation, poorly controlled cardiovascular disease, limb amputation, osteoporosis, orthostatic or postural hypotension); a recent fall that required physician evaluation (emergency department, urgent care, hospitalization) within the past year; use of an assistive device (or person) for walking, standing, or balance; and current use of a structured exercise regimen defined as participation in a regular exercise program consisting of more than 60 minutes per week in total.
Participants were recruited from rural areas in Wyoming and Nevada as a sample of convenience using snowball recruitment strategies and public media from November 2017 to December 2018. Of the 17 individuals who were formally screened, 15 matriculated into the study and all 15 completed the 8-week outcomes (Fig1). Fifteen individuals with PD participated (mean age 73.3 (range 57–93) years; 7 males, 8 females; mean disease duration 9.3 (range 1–22) years) (Table 1). All participants noted some technology experience prior to this telehealth study, with the most common experience being a cellphone. Thirteen of the 15 participants completed the 6-month follow-up (Fig1).
Table 1: Descriptive statistics of study participant characteristics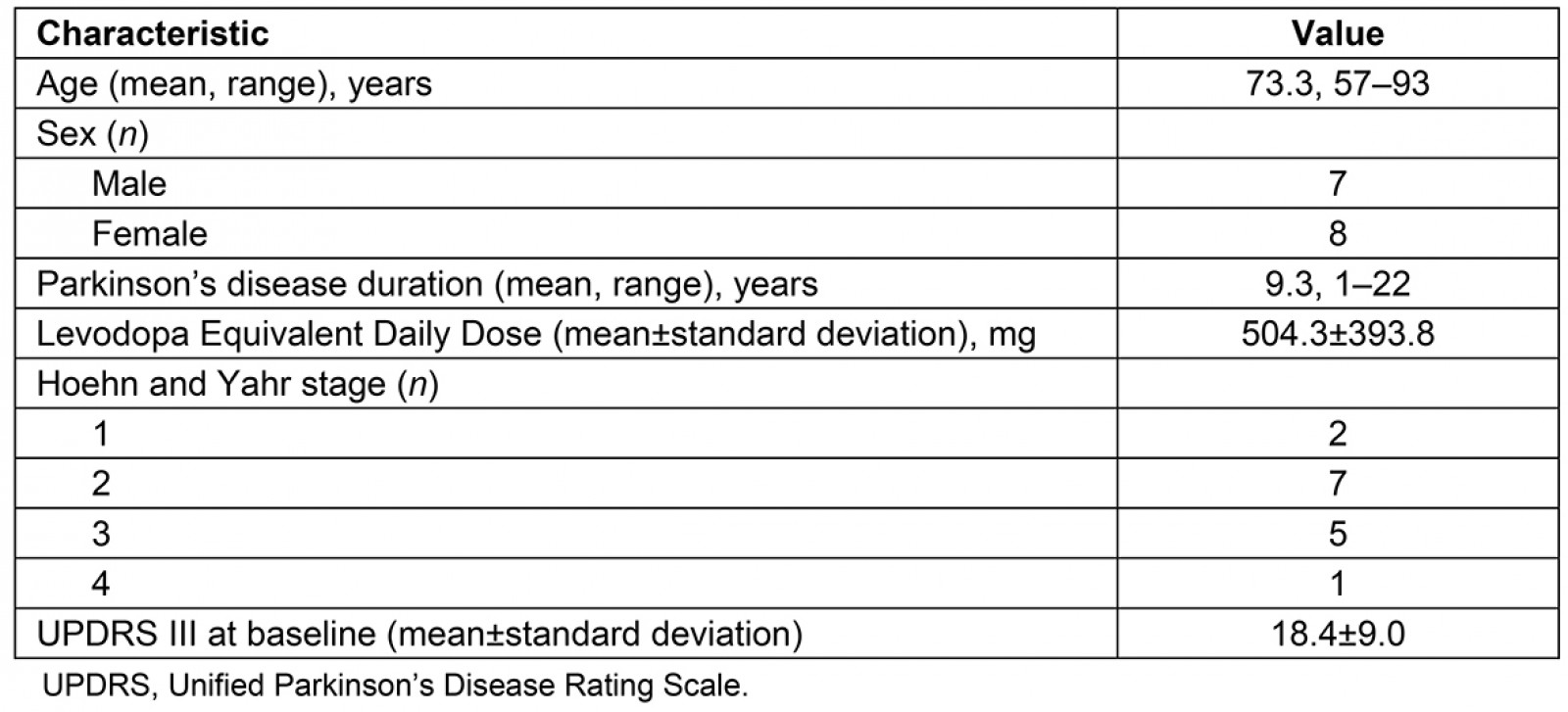
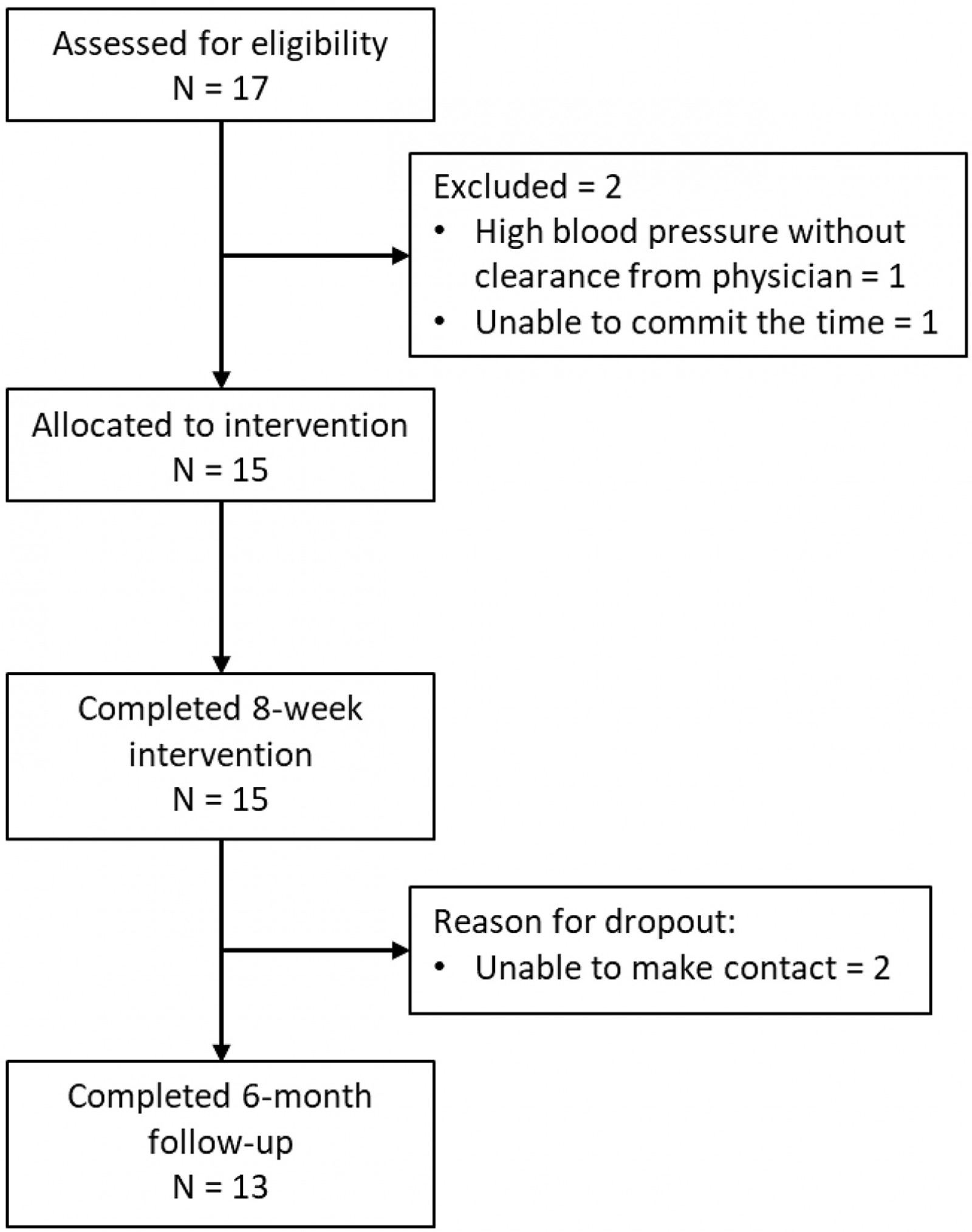 Figure 1: CONSORT flow diagram.
Figure 1: CONSORT flow diagram.
Sample size
For the first and second aims (feasibility and safety), a sample size of 20 was estimated for this study. This was based on the feasibility outcome of interest: participation rate. For a sample size of 20, a participation rate of 80±18% with a 95% confidence interval was estimated36,37. Based on an estimated 12% dropout rate from the sample population and type of study35, 88% was assumed as the actual participation rate for this study. With this in mind and the sample size justification, 80% power was calculated for this study. For the third aim (signal of efficacy), using the two-sided paired Wilcoxon Signed Rank module (PASS v20.0.3; NCSS; http://wwwncss.com/software/pass)), a sample size of 16 from an estimated rural PD population of 1000 from both Wyoming and Nevada would achieve 80% power to detect a mean of paired differences of 0.4 with an estimated standard deviation of paired differences of 0.5 and with α=0.05 assuming a normal distribution of paired differences.
Coordinated telehealth intervention
This 8-week telehealth program was coordinated by two study coordinators (one PD nurse and the other an assistant study coordinator), who both acted as liaisons to the participants and scheduled all of the treatment sessions and followed up with the participants weekly. The coordination of the study was an important feature as it was requested by people with PD from previous work of the authors18. Regarding their health care, participants from the previous exploratory studies described the burden of making several appointments with several providers and not having a single contact person. The authors honored this by having the study coordinators serve as the main contact to answer questions, schedule providers, troubleshoot technology problems, monitor adverse events with participants and providers, and conduct weekly check-in appointments.
All three parts (speech therapy, physiotherapy, and pharmaceutical care) of the 8-week telehealth program were administered by licensed healthcare providers (four speech-language pathologists, two physiotherapists, and two clinical pharmacists) using Zoom (Table 2). None of the healthcare providers were investigators of this study and all were blinded to the specific aims of the study. All participants received laptop computers equipped with all necessary software and capabilities to participate in their telehealth therapy sessions. Participants provided their own internet access. Participants received the intervention from a single healthcare provider within each of the three parts of the telehealth program. The coordinated telehealth program required approximately 8 hours of time from each participant per week of the 8-week study.
Table 2: Treatment overview for each of the three interventions over the 8-week program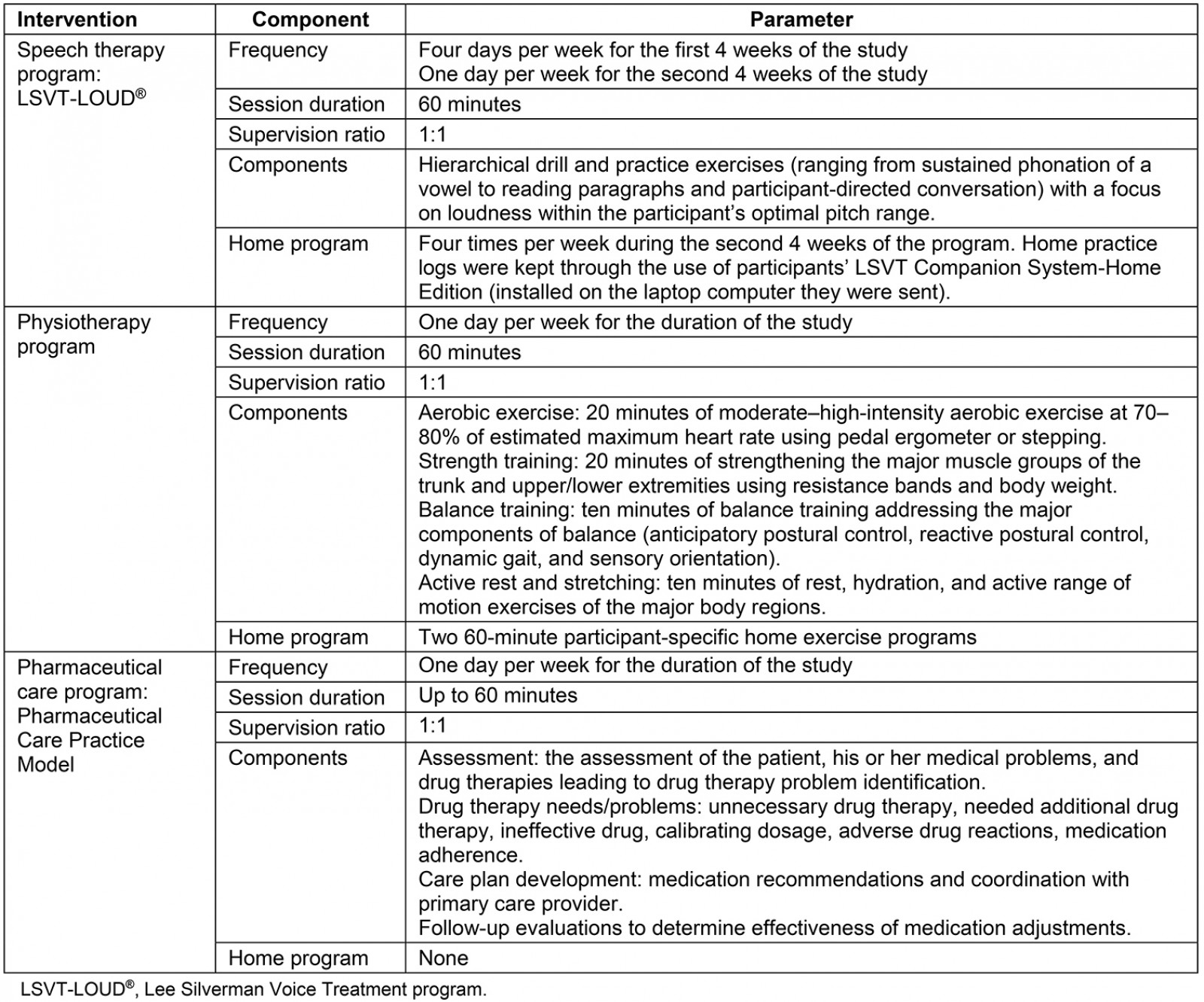
Speech therapy
All speech treatment sessions were consistent with the Lee Silverman Voice Treatment (LSVT-LOUD®) program38-45. Participants were sent the laptop computer previously mentioned with the LSVT Companion software (client version) already installed. To ensure treatment fidelity, all treating speech-language pathologists were trained on this regimented treatment protocol through LSVT® Global and also received online training to deliver LSVT via telehealth (eLOUD). Additionally, they all used the LSVT Companion software (clinician version) to conduct the speech therapy sessions. Participants were also sent a measuring tape and a microphone to ensure consistent distance and recording fidelity.
The speech therapy program consisted of four 60-minute sessions per week for the first 4 weeks of the program (Table 2). Session activities consisted of hierarchical drill and practice exercises (ranging from sustained phonation of a vowel to reading paragraphs and participant-directed conversation), focusing on sustaining intensity/loudness within the participant’s optimal pitch range. Specific tasks were chosen by the speech-language pathologists to individually tailor this program, based on the type of communication and communication partners the person with PD had, as well as the participant’s interests and preferences. During the second 4 weeks of the program, participants met with the speech-language pathologist once per week but were also instructed to complete the LSVT home practice regimen, which consisted of three additional, non-supervised practice sessions per week (4 days total)41. The participants were instructed to complete their home program with the Companion software if possible. Otherwise, they were asked to practice offline, as this is a standard aspect of the LSVT treatment.
Physiotherapy
The physiotherapy part of the program was administered by neurologic physiotherapists who had experience and expertise treating patients with PD. To ensure treatment fidelity, both treating physiotherapists were trained by the primary investigator on the program, which was based on a previously published program35. Prior to beginning the study, each participant received a pulse oximeter for safety monitoring, a cycling pedal ergometer for sitting aerobic training, and elastic bands to use for strength training. Physiotherapists supervised the real time, one-on-one, 60-minute physiotherapy program once per week for the 8 weeks. Participants were also assigned a participant-specific home exercise program to complete two additional 60-minute sessions per week. As this was a pragmatic study, physiotherapists tailored the intensity and the modality of the exercise within the study parameters (Table 2). This flexibility allowed the physiotherapists to use their clinical judgement to tailor the exercises to the needs and capabilities of individual participants and to address the common motor impairments associated with PD. Because of the telehealth format, a primary focus of this physiotherapy was safety, particularly with balance training. All exercises that put the participant at risk for a fall were conducted with the participant’s hand at a counter or chair, or in a sitting position when necessary for safety.
Pharmaceutical care
The pharmaceutical care arm of the intervention was administered by clinical pharmacists with experience in PD pharmaceutical care. All medication management sessions were scheduled for up to 60 minutes (ranging from 15 minutes to 60 minutes, depending on the number of medications taken by the participant and the number of drug therapy problems identified) per week for the 8-week study (Table 2). Each week, the pharmacist worked with the participant to identify potential drug therapy problems (eg unnecessary drug therapy, necessary additional drug therapy, ineffective drugs, calibrating dosage, adverse drug reactions, medication adherence). The pharmacist would then make recommendations to the primary care provider regarding adjustments if needed46. The pharmaceutical care sessions were participant-focused and generally covered the Pharmacists’ Patient Care Process as outlined in the Pharmaceutical Care Practice model46,47. The three major steps in the Patient Care Process are the assessment of the patient, his or her medical problems, and drug therapies leading to drug therapy problem identification; care plan development; and follow-up evaluations.
Outcome measures
Outcomes for first aim (feasibility) and second aim (safety): The main outcome of the first aim was the number of sessions completed by each participant. Feasibility was based on clinical experience and research in the same population by the research team35; it was assessed using attendance and attrition or dropout rate during the 8-week intervention and satisfaction (dichotomous (yes or no) about whether they felt the intervention was useful and helpful) at the 6-month point. Dropouts were operationally defined as those who stopped coming to treatment sessions and/or did not complete the 8-week measurement point. Based on previous research, less than 15% attrition was considered feasible35. For satisfaction, the threshold for feasibility was 90%. To be considered ‘feasible’, the threshold was that 80% of participants would have attended at least 80% of the treatment sessions over the 8-week trial. To collect these data, the study coordinators contacted participants weekly to determine the numbers of sessions completed. In addition, the study coordinators recorded the frequency and nature of adverse events during the 8 weeks of the program (second aim).
Outcomes for second aim (signal of efficacy): A signal of efficacy for the 8-week telehealth program would be determined if there was a positive trend toward improvement across the outcome variables below. Data for signal of efficacy were collected before and immediately after the program in the ‘on’ PD medication state in the following domains.
Overall outcome – disease-specific quality of life For an overall assessment of the program, quality of life was assessed using a disease-specific questionnaire, the PD Questionnaire-39 (PDQ-39)48. The PDQ-39 is a 39-item self-report quality-of-life scale for the following eight different dimensions: mobility, activities of daily living, emotional wellbeing, stigma, social support, cognitions, communication, and bodily discomfort. Each of the 39 items ranges from 0 (‘never’) to 4 (‘always’). The overall score and subsection scores are calculated by taking the means of each item divided by the total for that section, thus converting the score into a percentage, with higher percentages equating to more disability.
Speech therapy To determine the signal of efficacy for speech therapy, the Communicative Effectiveness Survey (CES)49-51 was used. It is a self-report rating scale designed to measure communication effectiveness in various life situations. The CES is an eight-question scale using a four-point Likert scale (1=’not at all effective’; 4=’very effective’) on how the participant’s social participation is affected by their speech and communication. The scores range from 8 (‘not effective’) to 32 (‘very effective’). For acoustic data outcomes, vocal sound level intensity was assessed using the LSVT Companion software. To acquire acoustic data, participants were asked to perform a prolonged vowel phonation (‘ah’) at least 10 times, as loudly as possible within quality parameters for as long as possible. The average sound pressure level (dB SPL) and duration for each try was then averaged across the 10 or more tries. From these two measurements, a third outcome, ‘work’, was derived by multiplying the average SPL by the average duration. These data were collected by the treating speech-language pathologists via the clinician version of the LSVT Companion software.
Physiotherapy For a signal of efficacy in the physiotherapy domain, one performance-based measure was collected by the treating physical therapists, 30 second Sit-To-Stand (30STS) test52. The 30STS test measures how many times the participant can stand from sitting in 30 seconds. In addition, data from the Parkinson’s Fatigue Scale53, a measurement of disease-specific fatigue, was collected by the nurse study coordinator. This scale measures 16 items using a five-point Likert scale for each item. Scores are averaged and range from with a low score of 1 (‘low fatigue’) and a high score of five (‘high fatigue’). To assess disease-specific motor function, the clinician-scored Movement Disorder Society Unified PD Rating Scale Part III (MDS-UPDRS III)54-58 was collected by the nurse study coordinator, who had been trained to administer the scale and who had administered the scale hundreds of times over many years of PD clinical practice. The MDS-UPDRS III has 18 items with scores ranging from 0 (‘normal’) to 4 (‘severe’). Two items that could not be assessed using telehealth were removed (items 3.3 (rigidity) and 3.12 (postural instability)). Thus, 16 of the 18 items were scored. A score of 0 indicates normal function whereas a score of 64 (the highest possible score) is suggestive of severe motor deficits.
Pharmaceutical care The main outcome for the pharmaceutical care intervention was adherence, measured through the gold standard of pill count, as described elsewhere59-64. This outcome was measured by the nurse study coordinator and consisted of percentage adherence to the prescribed PD medications.
Data analysis
Data were analyzed using SAS software v9.4 (SAS Institute, http://www.sas.com) and α=0.05. For the first aim (feasibility) and second aim (safety), the number of completed sessions, dropouts, and the number of participant-reported adverse events were tabulated. Participant satisfaction was tabulated from the dichotomous response (‘yes’ or ‘no’) about whether the coordinated program was useful or helpful to them. For the third aim (signal of efficacy), because of the small sample size the data were analyzed with non-parametric, within-group comparisons using Wilcoxon Signed Rank tests for each of the aforementioned pre and post outcomes. Cohen’s D ((meanpre – meanpost)/standard deviationdifference) for the pre and post difference was used to determine effect sizes for each outcome. For the 6-month outcomes, the Wilcoxon Signed Rank Test was also utilized.
Ethics approval
Participants consented under University of Wyoming Institutional Review Board approval (Protocol #20171030MH01724).
Results
Feasibility
For feasibility, there were no dropouts over the 8-week intervention. However, at the 6-month follow-up, the research team was unable to contact 2 of the 15 participants. All 15 participants of the 8-week intervention completed more than 80% of their scheduled treatment sessions (Table 3). At the 6-month follow-up, all 13 participants were satisfied with the intervention and deemed the study as useful or helpful to them.
Table 3: Session attendance for different intervention components of the study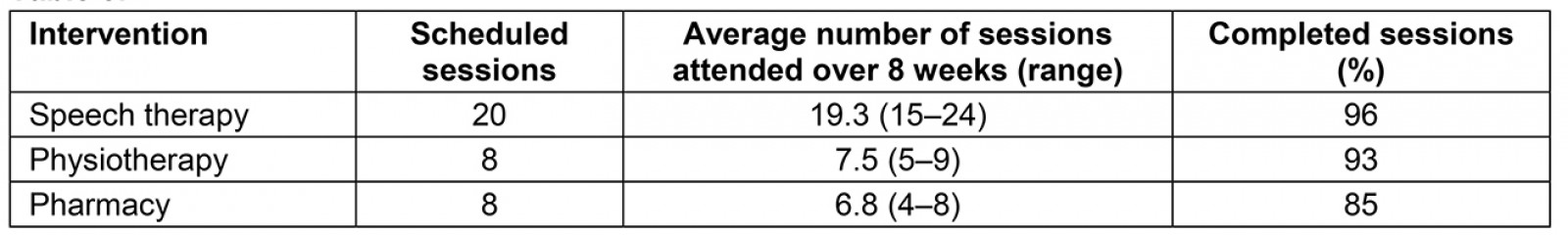
Safety
There were no serious adverse effects for any of the speech therapy, physiotherapy, or pharmaceutical care treatment over the duration of the study. For speech therapy, eight participants reported nine instances of adverse events (six hoarse voice, one sore throat, one strained neck, one coughing more than usual). However, the treating speech therapists only noted three participants with one adverse event each (dry mouth, voice cracking a lot, and extreme vocal fatigue). For physiotherapy, two participants reported two adverse events (strain/sprain and muscle soreness), both of which resolved without any intervention. The treating physical therapists did not report any adverse events. For pharmaceutical care, there were no medication-related adverse events. Of all of the treatment sessions conducted for all of the participants, there was a participant self-report of an adverse event in only 0.9% of the sessions.
Signal of efficacy
Outcomes across the four domains (quality of life, speech therapy, physiotherapy, and pharmaceutical care) are presented in Table 4. Effect sizes for those variables are detailed in Figure 2. Based on Wilcoxon Signed Rank Test results, the two outcomes that were statistically significant from pre to post were the 30STS (p=0.0039) and acoustic work (ie intensity times duration, p=0.0309; medians and interquartile ranges (IQR) are in Table 4). Because the telehealth LSVT-LOUD training stopped after 4 weeks and transitioned to a home program, interim analyses of the outcomes from pre to the 4-week measurements were conducted for the acoustic data: median volume changed from 78.7 dB (IQR=4.9) to 80.1 dB (IQR=7.6) (p=0.2011), median duration changed from 9.9 s (IQR=2.8) to 11.5 s (IQR=5.3) (p=0.0214), and acoustic work changed from 756.0 dB.s (IQR=198.4) to 876.3 dB.s (IQR=455.5) (p=0.0089) (Table 4). At baseline, 2 of 15 participants were more than 80% adherent to their PD medications. Although the pharmacists reported that all participants were adherent to their PD medications for the duration of the 8-week study, after the intervention only 4 of 15 were more than 80% adherent to their PD medications.
Table 4: Means and medians for each outcome variable associated with quality of life, speech therapy, and physiotherapy†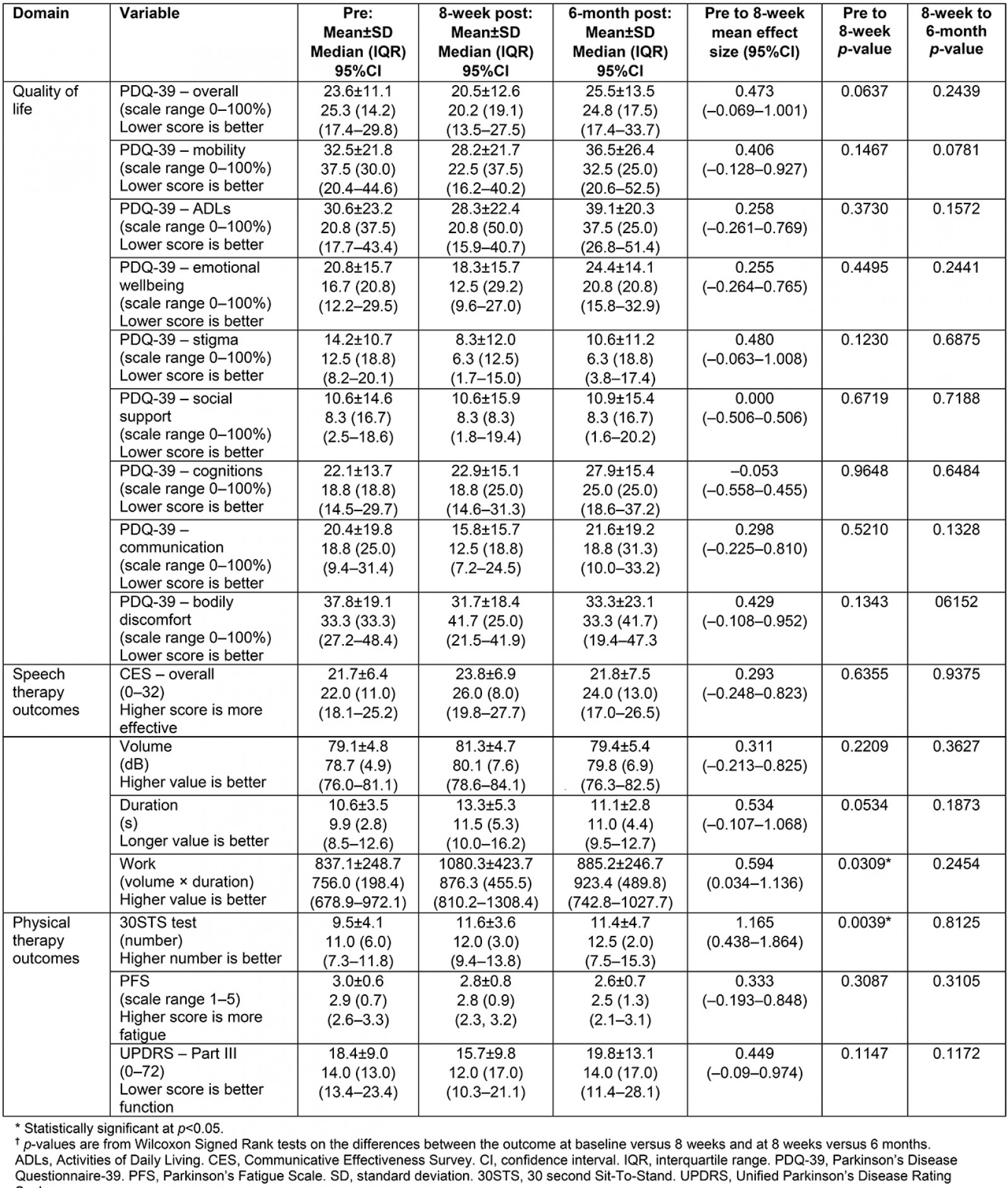
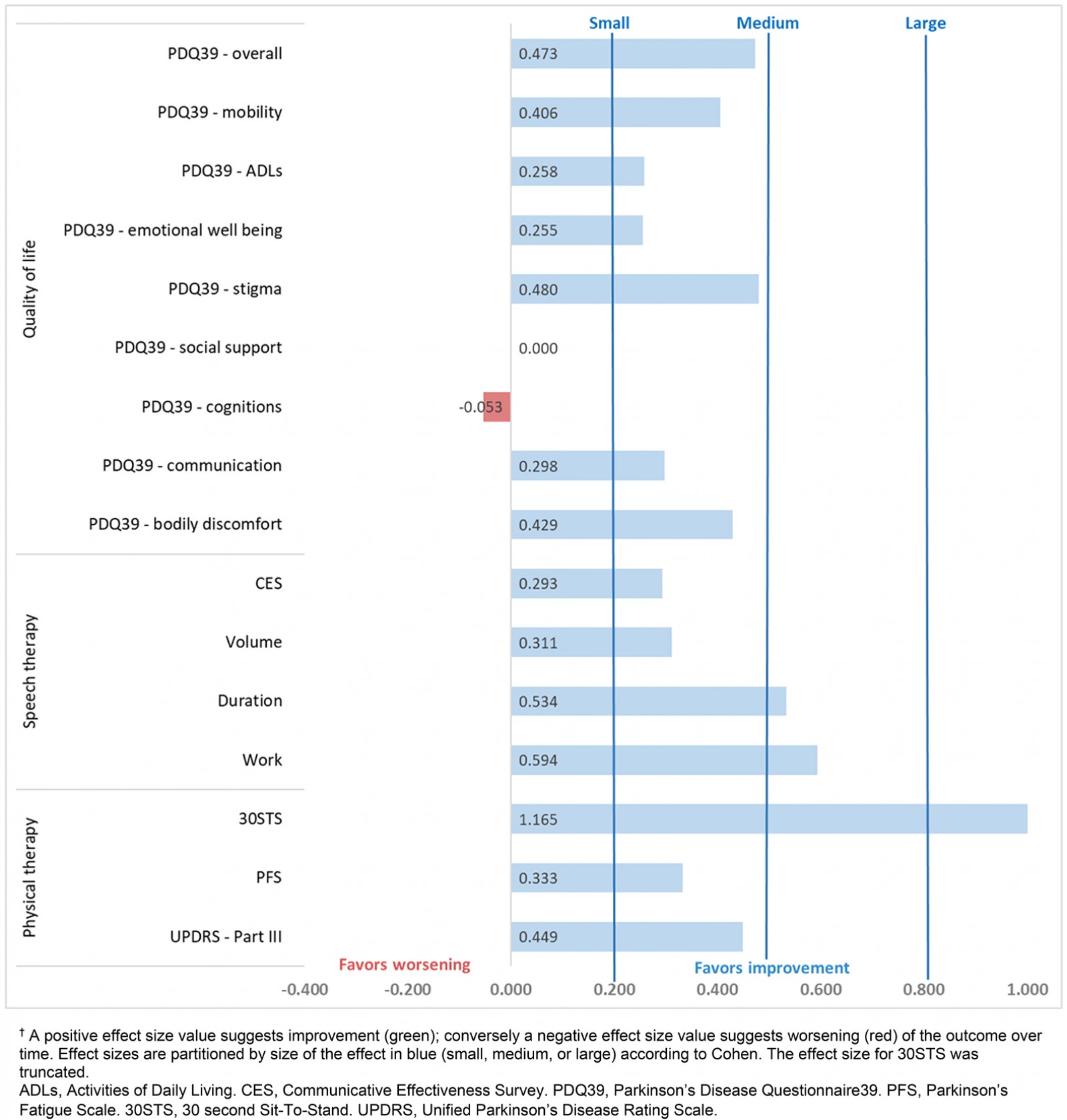 Figure 2: Effect sizes (Cohen’s D for paired measures65)† for the pre and post scores for the 8-week intervention.
Figure 2: Effect sizes (Cohen’s D for paired measures65)† for the pre and post scores for the 8-week intervention.
6-month follow-up
There was no evidence of statistically significant changes from the 8-week measurement to the 6-month follow-up for any of the outcome measurements.
Discussion
Results of this exploratory pilot study suggest that an 8-week coordinated telehealth program, consisting of speech therapy, physiotherapy, and pharmaceutical care, was both feasible and safe for people with PD living in rural areas. Additionally, there was a signal of efficacy that this telehealth program may be associated with changes that favor improvement across a majority of the outcome variables. However, due to the design of the study, causative inference due to the intervention should be cautioned. Future research using a more rigorous and controlled design of this promising telehealth program for people with PD in rural areas is warranted.
Although there were technology challenges with the intervention (detailed in the limitations section), the feasibility of this coordinated program was sufficient enough to warrant continued study in this area. Attendance at the required sessions was excellent for all participants and there were no dropouts over the 8-week program. Participants attended 96% of the scheduled speech therapy sessions, 93% of the scheduled physiotherapy sessions, and 85% of the scheduled pharmaceutical care sessions. There were no dropouts during the study and all who were interviewed at the 6-month follow-up were satisfied with the program. Collectively, these feasibility results are consistent with the feasibility reported in other telemedicine management programs12,14, telehealth exercise programs66-69, telerehabilitation balance training programs70, and LSVT speech therapy71-75 for people with PD. Although all of these studies reported the feasibility of telehealth for people with PD, they were all monodisciplinary studies and not multidisciplinary like the present study. Thus, the results of the present study are the first, to the authors’ knowledge, to offer preliminary evidence that a coordinated, multidisciplinary approach is feasible in PD.
All of the adverse events in this study were minor and resolved without any medical attention. Moreover, all of them are considered Grade 1 adverse events (Common Terminology Criteria for Adverse Events), which are the most minor adverse events in the spectrum76. Importantly, these minor adverse events were consistent with the frequency and types of minor adverse events that are experienced in typical in-person rehabilitation programs. Importantly, there were no falls, which was the biggest a priori concern for the physiotherapy portion of the program. Both of the physiotherapy adverse events are similar to the types of minor adverse events that happen with physiotherapy and exercise programs77,78. The speech therapy adverse events are consistent with the frequency and type of minor adverse events expected with in-person LSVT-LOUD therapy38,79. Another primary concern of the research team was mitigating the possibility of a serious adverse event that would necessitate immediate medical care, because the participants were living in rural areas and would not likely have immediate access to emergency care services. Thus, the exclusion criteria were more extensive than those for a typical in-person research study, and this would certainly limit the generalizability. Additionally, the exercises in the physiotherapy portion were lower in intensity than what would typically be experienced in an in-person physiotherapy session. As there was no spotter, balance training tasks were not as challenging as would be experienced in an in-person physiotherapy session.
An important part of this pilot study was determining if there was a signal of efficacy for this intervention to determine if future research in this area was warranted. While there were only two statistically significant improvements among the 16 outcome variables, 14 of the 16 outcomes had small to medium effect sizes (Fig2). Collectively, these data suggest a signal of efficacy that warrants further investigation. In addition, these signal of efficacy results are consistent with the ‘in-person’ efficacy reported in systematic reviews and meta-analyses for speech language therapy80,81, LSVT-LOUD81,82, physiotherapy77,83, and exercise77,84-86 for people with PD. It should be noted that the meta-analysis by Yuan et al for LSVT-LOUD did not find a difference in therapeutic effect between in-person and online LSVT, which supports the notion that these programs are comparable82. Additionally, a recent meta-analysis in PD found that telehealth is effective at improving motor impairment in PD, which is also consistent with the findings of this study87.
Collectively, there is considerable evidence for each of the interventions used in the present study in an in-person monodisciplinary approach. The uniqueness of the present study is that it was not only conducted using a telehealth format but was also multidisciplinary and focused on coordinated care. This study provides preliminary evidence that the foundational, in-person therapeutic efficacy of the different intervention components in this study is potentially transferrable to a telehealth format. If this were to hold true in larger scale trials, then it would be potentially meaningful and impactful in helping address each of the aims in the Triple Aim to improve the US healthcare system23. The Triple Aim as applied to the present study includes:
- improving patient care and experience – decreasing travel time and increasing accessibility to PD specialists
- improving population health – integrating and optimizing care for people with PD in rural areas
- reducing healthcare costs – using technology that reduces outpatient visits and allows access to experts who can proactively manage issues early for those living in rural areas3.
Limitations
One of the strengths of the present study is that this was a program involving an integrated coordination of multidisciplinary care for people with PD living in rural areas without access to PD specialists. However, the typical PD care team clearly involves other disciplines (eg occupational therapist, nutritionist, movement disorders neurologist, psychologist, social worker) that were not included in this study. Future coordinated, multidisciplinary telehealth approaches for people with PD should consider integrating other important PD care team members, as has been described in the literature3,88. Another limitation of the present study is that enrollment targets were not met within the recruitment and funding window. Subsequently, each of the aims were likely underpowered and, therefore, the results and generalizability of the findings should be interpreted with some caution. Additionally, since ‘commitment to attend scheduled sessions’ was intended to create a more committed participant pool, it may have unintentionally contributed to a biased sample and, therefore, limit the generalizability to those willing to participate in a trial like this.
While an extended discussion of the technology challenges would not be appropriate here, it should be noted that there were some logistical and technology related challenges (eg audio/video problems, slow internet connections, participant inexperience/intimidation by laptops/telehealth technology, laptop rebooting/updates during telehealth sessions). These types of challenge were expected, and effort was made to mitigate them; however, they remained a primary issue over the duration of the study. While all participants were tested in the ‘on’ PD medication phase, there was a lack of control of assessment occurring at the peak ‘on’ phase times and this may have impacted performance with the outcome variables. Another limitation was that, although home exercise program adherence was tracked and encouraged by the treating speech and physical therapists, it was not formally tracked for reporting purposes. Lastly, the pill count data was taken by the nurse study coordinator rather than by a pharmacist, and this resulted in some inconsistencies, which precluded a more detailed report of participant medication adherence. In light of this and other confounding factors related to changing medication regimens over the duration of the study, it is recommended that future research in a coordinated, multidisciplinary study like this utilize a medication management run-in design, wherein medication management is the first phase and, once the PD medications have been sufficiently stabilized, the rest of the coordinated intervention is implemented.
Conclusion
An 8-week coordinated telehealth program, consisting of speech therapy, physiotherapy, and pharmaceutical care, was both feasible and safe for people with PD living in rural communities. Additionally, results from this pilot study suggest that there was a signal of efficacy favoring improvement across a majority of the outcomes for this telehealth program. However, because this was a single cohort design, causal inference should be cautiously interpreted. Future research using a more rigorous and controlled design of this coordinated telehealth program for people with PD in rural areas is warranted.
References
You might also be interested in:
2021 - Researching ‘others’
2020 - Patient and provider perspectives on eHealth interventions in Canada and Australia: a scoping review





