Introduction
The Indigenous population of Brazil comprises 817 963 people, corresponding to 0.4283% of the total Brazilian population. According to the latest demographic survey conducted in 2010, Indigenous people are distributed in both rural and urban areas of the country1,2. Xingu Indigenous Park (XIP) was created in 1961 to protect the physical and cultural aspects of the Indigenous peoples who live there, guaranteeing environmental preservation and sheltering Indigenous groups threatened with extinction. It is the first and the largest Indigenous land to be recognized in the country, protecting 16 ethnic groups. XIP is located in the central area of Brazil, south of the Amazon and north-east of Mato Grosso state. According to the Indigenous Health Information System, in 2013, the Indigenous population of XIP totaled 6589 inhabitants distributed in four regions: Upper, Middle, Lower, and East Xingu2.
In recent decades, the exposure of this population to different infectious agents has increased, as has contact with non-Indigenous people living in the vicinity, and the opening of new roads. Indigenous women are more exposed to risk cofactors for the development of cervical cancer, such as early sexual activity, multiple sexual partners, the non-use of condoms, and multiparity; additionally, it is common for Indigenous women to spend approximately 30 years in a prolonged reproductive period3. The Indigenous population is constantly dealing with shortages of medical care owing to restricted and limited access for outsiders to the Indigenous area. Organized activities such as STI screenings, prenatal care, and laboratory tests are not available4. Moreover, when treatment of Indigenous women does occur, a syndromic approach is taken owing to the lack of laboratory testing for STIs, as recommended by the Brazilian Ministry of Health5.
There is a high rate of cervical cancer among Indigenous women worldwide6. Oncogenic types of high-risk human papillomavirus (HR-HPV) are responsible for 99% of cervical cancers, which is the second most common cancer in underdeveloped countries7,8. HR-HPV16 and 18 are responsible for approximately 70% of cervical cancers9,10. Persistent HPV infections in women are also associated with genital warts, as well as vulva and vaginal cancers9,11.
Other STIs, such as Chlamydia trachomatis (CT) and Neisseria gonorrhoeae (NG), are the major causes of pelvic inflammatory disease and infertility in women12. CT and HPV have the same sexual transmission pathway with similar risk factors, indicating that a co-infection can act synergistically in the induction of cellular atypia13.
CT is responsible for the most frequent bacterial infections in the world, followed by NG, according to the WHO14,15. CT is commonly found in sexually active young people14, and in untreated women it can lead to pelvic inflammatory disease, fallopian tube complaints, infertility, chronic pelvic pain, and ectopic pregnancy16,17. Studies have demonstrated that the placenta and amniotic fluid can become infected, indicating a strong association with premature births and miscarriage18. A co-infection of CT and NG can also bring on these symptoms and disorders19. Furthermore, gonococcal infection has progressively developed resistance to the antibiotics that have been increasingly used in recent years, thus reducing the options for treatment. Asymptomatic cases of gonococcal infection are very common and can result in a fivefold increase in HIV transmission20,21.
The notification rates of CT and NG infections in Indigenous people in Australia have increased by 80% and 22%, respectively. The highest notification rates were in infections in Indigenous women aged 15–19 years, and those living in remote areas22. However, to the best of our knowledge, no previous studies have described the epidemiology, clinical features, and management related to STIs caused by CT and NG in Indigenous women from XIP. An investigation of these infections, together with the detection of the presence, and viral type, of HPV can significantly contribute to the understanding and control of STIs in the Indigenous population. Therefore, the present study aims to evaluate the lower genital tract infections caused by CT, NG, and HPV in the Indigenous women of XIP.
Since 2005, the Federal University of São Paulo – Paulista Medical School (UNIFESP-EPM), including a group of professionals from the Health and Environment Unit of the Department of Preventive Medicine/Xingu Project, and the Prevention of Gynecological Diseases Center (NUPREV) of the Department of Gynecology, has been working on the prevention, diagnosis, and treatment of diseases of the lower genital tract of sexually active women from XIP. The implementation of this cervical cancer prevention program has resulted in a reduction in the number of abnormal pap smears, showing that the strategies have been effective in controlling cervical intraepithelial neoplasia. This type of special attention offered by the university to this Indigenous population is unusual in Brazil. Indigenous communities must usually rely on unstable or absent financial resources or non-governmental organizations.
Methods
This cross-sectional study included 992 Indigenous women living in the Upper, Middle, Lower, and East Xingu regions of XIP who met the inclusion criteria: participants must have had or currently have sexual partners and must be aged more than 18 years. Pregnant women of any gestational age were excluded from the study.
Collecting and preserving the samples
The collection of cervical smear samples from the women was organized in two phases in 2018 owing to the difficulties accessing these areas. The first samples, collected in the first phase, were from women from Upper Xingu (n=499) and the second phase collection was from women from Lower, Middle, and East Xingu (n=493). The collections were carried out in health centers, schools, empty houses, or isolated areas according to the structure or availability of the community. The workflow diagram of the study is shown in Figure 1.
Patients were kept in a gynecological position, the Collins speculum was introduced vaginally, and, after exposure to the cervix, the cellular material was collected with a Rovers® Cervex-Brush® (https://www.roversmedicaldevices.com), which was used as suggested by the manufacturer. A SurePath liquid medium (https://www.bd.com/en-us/products-and-solutions/products/product-brands/surepath) was employed for sample storage and preservation.
For data analysis and to verify the infection index of CT and HPV, we defined the cut-off age as 25 years, maintaining an age balance between the CT screening guidelines (less than 24 years according to the Centers for Disease Control and Prevention and more than 25 years for cervical cancer screening according to the Brazilian Guidelines for the Control of Cervical Cancer5,23).
Cobas® 4800 for HPV test
HPV genotyping tests were performed at the Hospital de Amor pathology service, using the Roche cobas 4800 detection system (Roche, Branchburg, New Jersey, US). The cobas 4800 identified 14 types of HR-HPV in a single analysis, including the individual detection of HPV16 and HPV18, and the simultaneous detection of 12 HR-HPV types (31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68) that form a single group named ‘other types’ at clinically relevant levels of infection24. All protocols were performed according to the manufacturer’s instructions. Subsequently, all samples were carefully analyzed. To better describe the results, subjects were divided into three groups based on cobas 4800 genotyping data: an HPV16 group; an HPV18 group; and an ‘other types’ group that included women with an amplification of HPV 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, or 68.
Abbott RealTime CT/NG
The detection of CT and NG was performed at the retrovirology laboratory in the infectious diseases discipline at UNIFESP-EPM. All samples were tested with the Abbott RealTime CT/NG assay (Abbott Laboratories, Illinois, US), which used quantitative reverse transcription (qRT)-PCR detection for the pair CT and NG25-27. The assay consisted of two reagent kits: the Abbott RealTime CT/NG amplification reagent kit and the Abbott RealTime CT/NG control kit. DNA was extracted from each specimen using the Abbott m2000sp automated system, and qRT-PCR detection was performed with the Abbott m2000rt instrument for amplification and detection according to the manufacturer’s instructions. A negative control and two replicate samples of the cut-off control were used for each process. This system is a qualitative assay and the data were interpreted as previously described28.
Statistical analysis
The age of the women is presented by mean and standard deviation (mean±SD). For qualitative comparisons, Pearson’s χ2 test or Fisher’s exact test was used to analyze the differences among the frequencies of each variable. Statistical analyses were performed using GraphPad Prism 5.0 (GraphPad Software, California, US) and Statistical Package for Social Sciences v21 (IBM Corp.; https://www.ibm.com/products/spss-statistics). Statistical significance was set at p≤0.05.
Ethics approval
The present study was approved by the Ethics and Research Committee of UNIFESP-EPM under protocol number CEP 1753/06 and the National Research Ethics Commission under protocol number 14320, and was conducted in accordance with the ethical standards of the Declaration of Helsinki. Prior to the study, all patients agreed with and signed an informed consent form.
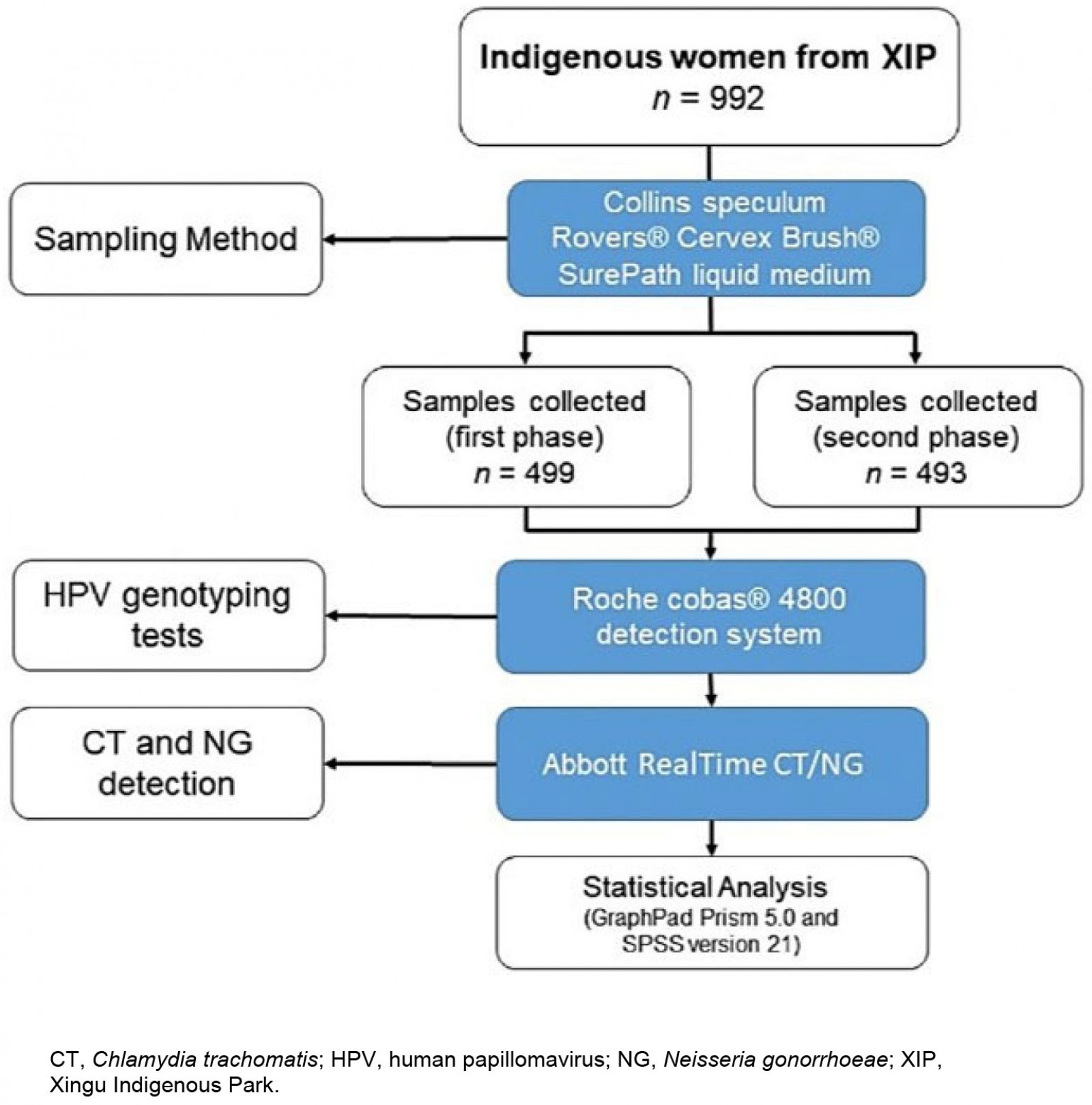 Figure 1: A flow diagram of the study design with details of the number of participants and diagnostic method used.
Figure 1: A flow diagram of the study design with details of the number of participants and diagnostic method used.
Results
The Indigenous women had an average age of 33.2±12.0 years, ranging from 18 to 75 years. In total, 992 samples were tested for HPV, of which 23 remained inconclusive and 18.2% (177 of 969) were positive for some kind of HR-HPV. The presence or absence of HPV (positive or negative) was also evaluated and distributed by age. Of the women aged ≤25 years (n=317), 26.8% (n=85) were positive and 73.2% (n=232) were negative for HPV. Of the women aged ≥26 years (n=652), 14.1% (n=92) were positive and 85.9% (n=560) were negative for HPV (p≤0.0001). It was observed that women aged less than 25 years had a higher frequency of HPV infection than older women proportionally (Table 1).
Furthermore, among HPV-positive women, 48% (85) were ≤25 years and 52% (92) were ≥26 years. The HR-HPV other-types group was most frequently found in women aged ≤25 years (72 women; 22.7%), while for those aged ≥26 years, 11.2% (73) of women were positive for this viral group (p≤0.0001).
Comparing the distribution frequency between HPV16, HPV18, and the other-types group, women infected with the other-types group were the majority (145 of 177 women; 81%). Only 6% (11) of women had HPV16 exclusively and 5% (8) had HPV18 exclusively. Co-infection of HPV16 and the other types was found in 5% (8) of women, and co-infection of HPV18 and the other types was found in 3% (5).
From looking at the viral distribution of HPV between regions (Fig2), it can be seen that 100% of HPV infections in East Xingu were the HR-HPV other-types group. In Middle Xingu, HPV infections included 96.6% (28) of the other-types group (either alone or co-infected with HPV16) and 6.8% (2 women) with HPV16. In Lower Xingu, the other-types group was represented in 95.5% (42) of infected women and HPV16 in 13.6% (6). Upper Xingu had 88.9% (80) positivity for the other-types group and 12.2% (11) for HPV16 (Table 2).
Regarding the analysis of CT detection, it was observed that 1.8% (18) of women were positive. The distribution analysis by XIP region showed that 38.9% (7) were from Lower Xingu, 27.8% (5) were from Upper Xingu, 22.2% (4) were from Middle Xingu, and 11.1% (2) were from East Xingu (Table 3). Women aged ≤25 years had a higher frequency of positive tests (55%) than those aged ≥26 years (45%).
In the CT-positive group, 33% of women had an HPV co-infection (28% had a co-infection with the other-types group and 5% had a co-infection with HPV16 and the other-types group (p=0.083)). We did not find any women who were positive for NG.
Table 1: Human papillomavirus distribution by age group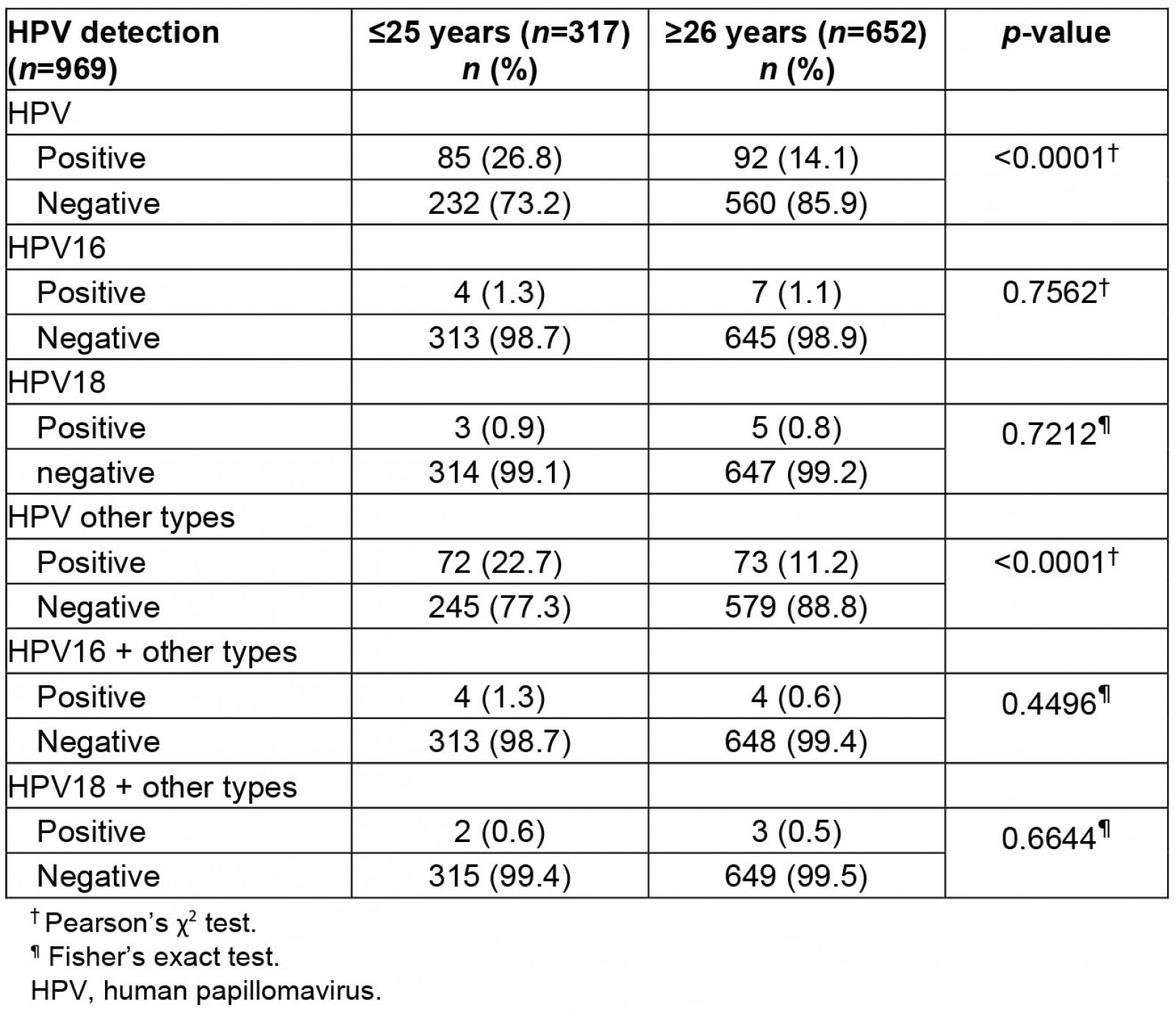
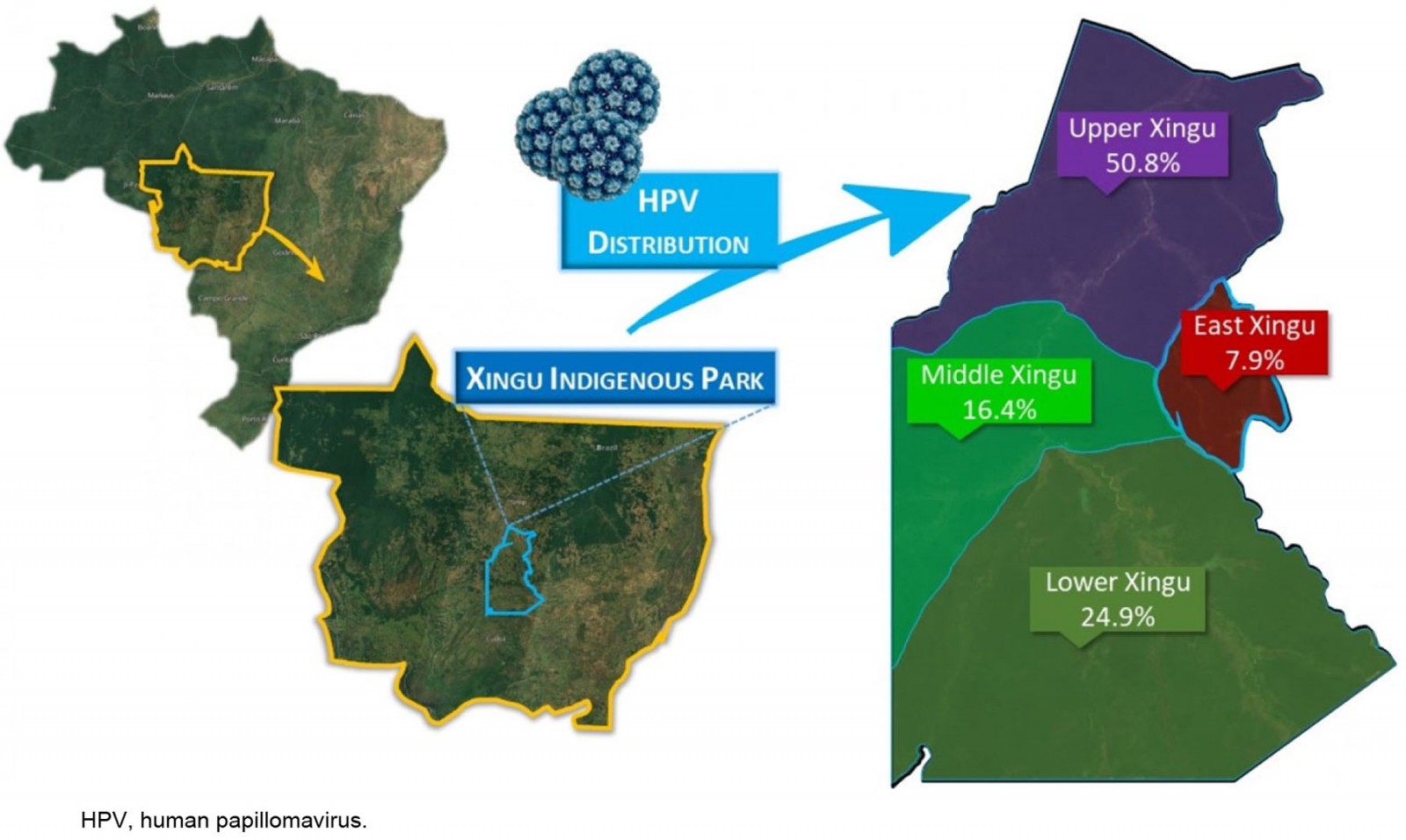 Figure 2: Location of the Xingu Indigenous Park and frequency of distribution of the high-risk human papillomavirus infections in Indigenous women by region (n=177).
Figure 2: Location of the Xingu Indigenous Park and frequency of distribution of the high-risk human papillomavirus infections in Indigenous women by region (n=177).
Table 2: Distribution of human papillomavirus positivity and viral type by Xingu region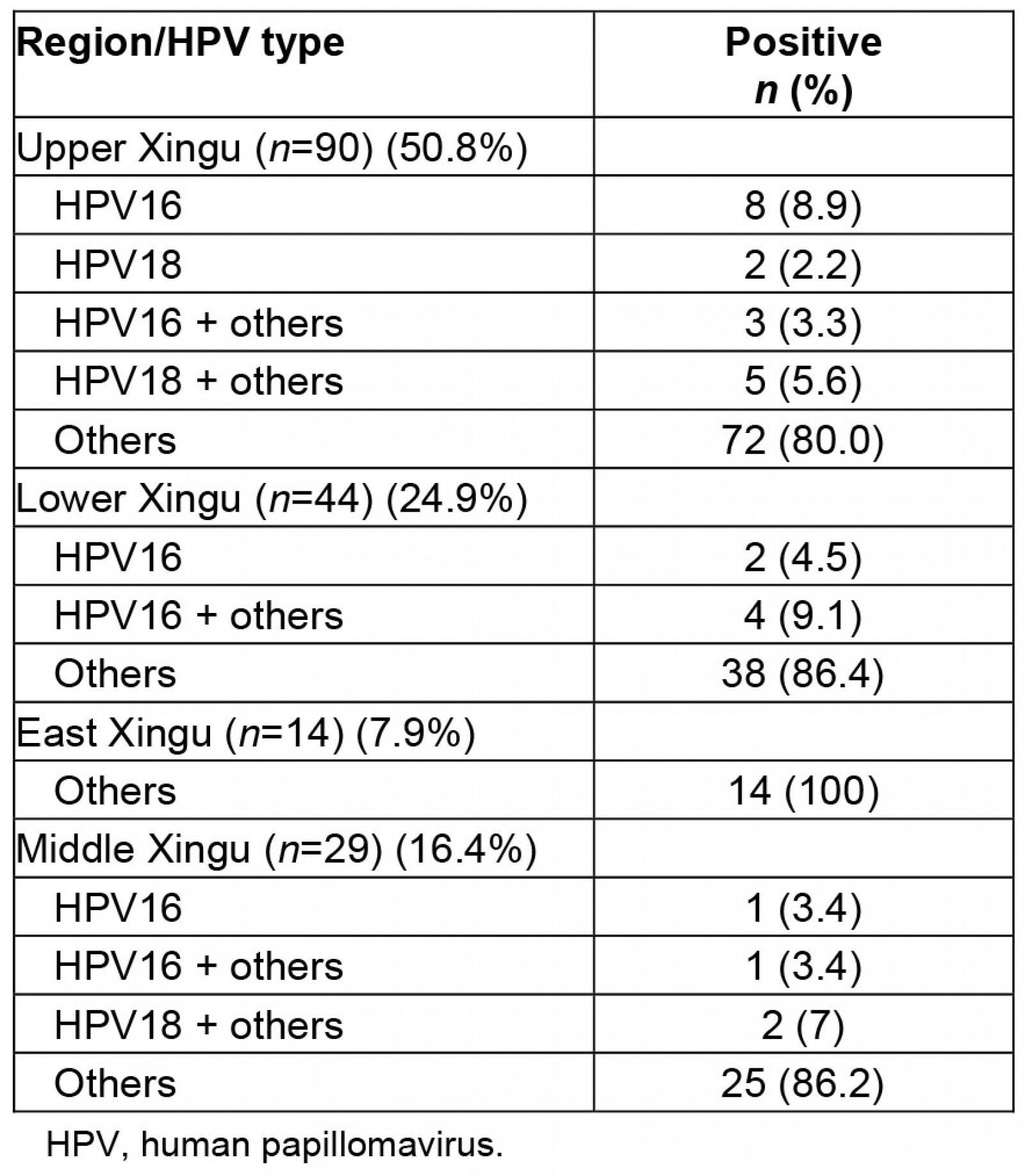
Table 3: Distribution of positive cases of Chlamydia trachomatis by Xingu region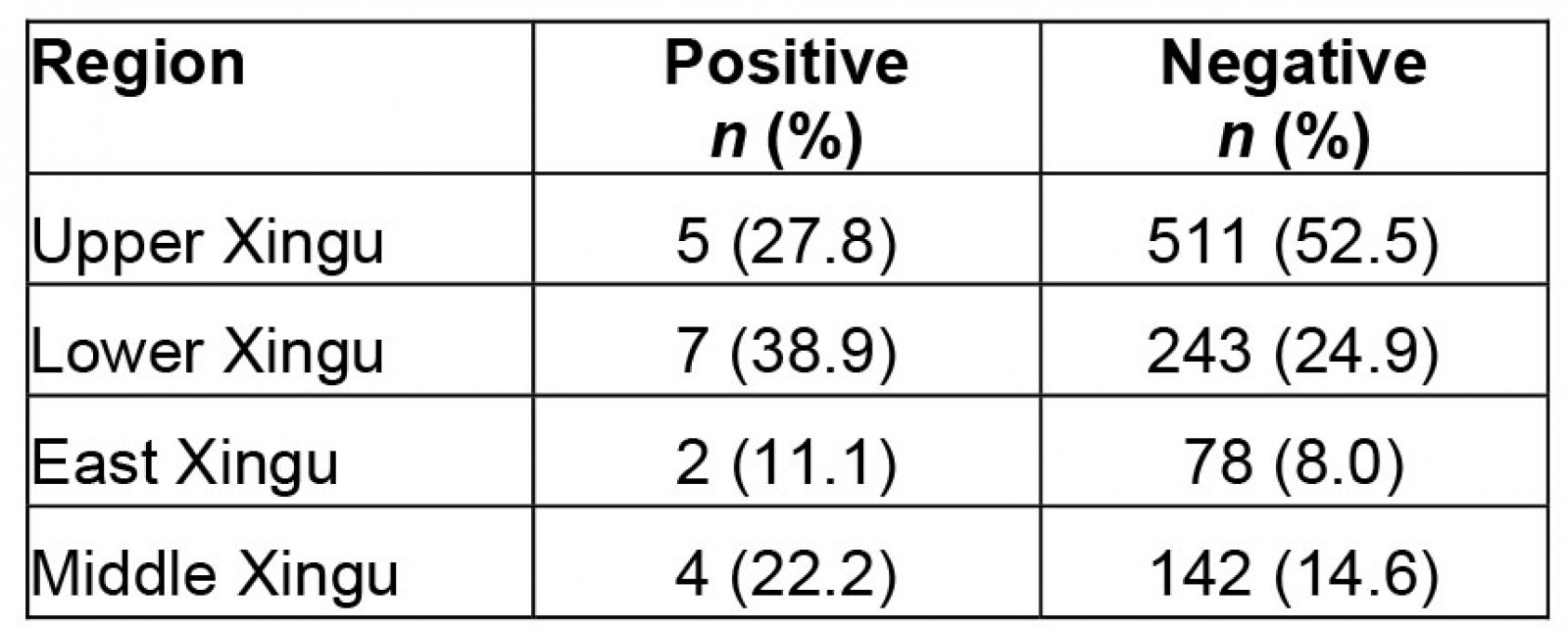
Discussion
Although a low proportion of squamous intraepithelial lesions have been reported in the XIP6,7, the present study revealed a high incidence of HPV infection (18.2% of women tested were positive). However, fewer than 5% of the infections were HPV16, contrasting with the most frequently reported findings in the literature29, which might explain the low frequency of severe cervical lesions reported by others6,7.
From these results it can be inferred that the female population studied in the XIP has a specific pattern of HPV frequency, a pattern that is also evident in other populations30.
The prevalence of HPV subtypes in Indigenous populations tends to differ from that in the general population31. One can speculate whether the pattern of HPV infection in XIP would lead to predictable or unpredictable outcomes regarding neoplastic progression, but there was no evidence to make a credible assumption about the potential association between these types of HR-HPV infections and neoplastic progression. At this point, the most acceptable hypothesis for the findings is a circumstantial laboratorial endpoint and cannot be validated in the present report; however, appropriate study designs in further projects may clarify this speculation.
It could be hypothesized that a factor leading to the relatively higher incidence of types of HPV other than HPV16 or 18 is the quadrivalent HPV vaccine distributed in this population, decreasing the risk of infection by the main types of HR-HPV, 16 and 18, but without ideal coverage for the other HR-HPV types. This suggests the potential added value and usefulness of implementing the nonavalent vaccine in populations with a prevalence of different HPV types. The HPV vaccine has been fully available for young women aged 9–13 years in XIP since 2014, and has substantially reduced the occurrence of severe cervical lesions32. The XIP is doing well in this regard, as experienced by well-trained health professionals who have been visiting the area regularly for many years, with a decrease since the cervical screening program began in the chance of serious cervical abnormalities and their most dire consequences developing. The current data demonstrate that well-organized and uninterrupted prevention minimizes the impacts of oncogenic HPV infection, and that the risks of neoplastic development can be reduced for these Indigenous populations33.
Epidemiological statistics related to vaginal infections in Indigenous female populations are not very clear in Brazil, or worldwide34. Part of the reason for this lack of information is the difficulties related to geographical access, as the logistics of transit to the places where these populations are located are very limiting. In addition, communication difficulties with the Indigenous women, who remain integrated in their daily lives and cultures in the villages, make interactions problematic, along with the difficulties associated with the provision of medical information and the necessary guidance on the appropriate use of initiatives to control sexually transmissible infectious diseases. This complex network of cultural variables requires careful planning to implement prevention strategies that are adapted to different populations.
The screening processes for cervical cancer in the XIP proposed by the UNIFESP-EPM, based on cytological analysis and clinical examinations, started in 2005 and have had significant results over the years in terms of a reduction in severe cervical lesions. Since then, the coverage rate has reached 90% of sexually active women aged more than 18 years; rates much higher than those found in many metropolitan cities35,36. The recommended age for the screening of cervical lesions in Brazil, and in many countries, is 25 years, because at younger ages, there may be a large number of HR-HPV-positive women whose infections will clear up spontaneously, without inducing cervical lesions15,18. An arrangement to reduce this age was undertaken by the Xingu Project due to the occurrence of high-grade lesions cases in women aged less than 25 years. Taking into account that Indigenous women often move to other tribes and have their names changed, it would be very difficult to monitor these patients, as they could avoid the clinical follow-up of serious infections for long periods. In addition, early sexual activity and multiparity are important risk factors for cervical cancer and are very common in XIP7,37,38.
As previously mentioned, of the total 992 women who participated in the present study, 18.2% were positive for some type of HR-HPV, and infection by non-HPV16 and non-HPV18 types was evident in 90% of the positive women. Furthermore, women aged ≤25 years were the most affected by non-HPV16 and non-HPV18 types, which was expected due to the early sexual activity in this population. It is worth mentioning that in a previous study conducted by Freitas and colleagues (2016), this population showed a significant prevalence of HPV52 in histological sections of high-grade lesions, which is in agreement with our supposition39. When analyzing the viral distribution among the regions, 100% of HPV infections in East Xingu were of non-HPV16 and non-HPV18 types. In Middle Xingu, the same non-HPV16 and non-HPV18 types totaled 96.6% of infections, with 6.8% of infections of HPV16. Of women positive for HPV in Lower Xingu, 95.5% had non-HPV16 and non-HPV18 types and 13.6% had HPV16. In Upper Xingu, the other-types group accounted for 88.9% of HPV infections and HPV16 accounted for 12.2%.
Despite the great success in preventing cervical cancer in the Indigenous people of XIP by NUPREV and the Xingu Project, previous to this present study, there has never been any type of test offered to the women to identify STIs. In the present study, we performed molecular tests for the viral genotyping of HR-HPV, CT, and NG (which are among the main STIs affecting the world), providing for the first time the characteristics of an important epidemiological profile distinguishing these infections in the women of Xingu12,40. According to the Centers for Disease Control and Prevention, the screening of CT infections should be started younger than 18 years, ideally at 14–24 years23. In the XIP, 1.8% of women were positive for CT. This finding conflicts with that of Ishak and colleagues (2001)41. They found 48.6% of immunoglobulin G antibodies to CT in 48 Indigenous communities in the Amazon region. Although comparison of study results for genital infections among American Indigenous women is frequently not feasible because of differences in methodologies, the studies have produced interesting and important data. For example, regarding CT infection, Gabster and colleagues reported a very high prevalence among adolescents in Panama (15.8%)42. In another study published by the same authors, a remarkably high prevalence of sexual infections in Indigenous adolescents from Panama was observed, in which bacterial vaginosis and HPV accounted for 42.9% and 33.2%, respectively43.
In several studies from around the world, a higher rate of CT infection has been reported in women up to age 25 years44, which was also found in XIP. In the present study, we found the highest CT infection rate in the Lower Xingu region (38.9%), followed by Upper Xingu (27.8%), Middle Xingu (22.2%), and East Xingu (11.1%). No woman was found to be positive for NG, an unexpected fact that warrants further attention. Co-infection of CT and NG is not uncommon, leading us to wonder about the potential implications of the absence of NG in the XIP population45,46.
Curiously, 33% of the women positive for CT had co-infections of HPV, all of which were related to non-HPV16 and non-HPV18 types. This index is especially interesting since it has been reported that this co-infection is more frequent in women with high-grade squamous intraepithelial lesion than in those who have only an HR-HPV infection47.
Despite the data produced in the present study, it is important to note that understanding of the XIP population would benefit from analysis of a more comprehensive panel of genital infections, and a more inclusive and specific questionnaire on the sexual habits of the women studied (although this would be difficult for cultural reasons). The language barrier was a factor that also limited data collection from the questionnaires, since many different languages and dialects exist in the XIP. Another factor that limited the study development was cost; the study had access to scarce resources and relied on the donation of materials.
Conclusion
The frequency of HR-HPV was at a high level for a population living in closed Indigenous communities, even though they are close in many ways to communities of other than Indigenous people. We observed a notable frequency of CT and an intriguing absence of NG. Finally, no evidence was found of a potential interrelationship between the three etiologic agents for a combined pathogenic action, also excluding a subsequent synergy between CT infections and HPV. This study provides data on the rates of HPV, CT, and NG in the Indigenous women of Xingu, but it does not examine the relationship between infections with these agents and the development and severity of related diseases.
Acknowledgements
Thanks to Juliana Galinkas and Vivian Vaz for methodological contributions.
Statement of funding
This study was financially supported by the Public Ministry of Labor Campinas (Research, Prevention, and Education of Occupational Cancer, Brazil). The Coordination Agency for the Improvement of Higher Education Personnel (CAPES) provided financial assistance during the execution of this project. Abbott Laboratories donated PCR kits for CT and NG; Rovers donated the endocervical brushes. Hospital de Amor, Barretos, Brazil, provided us with the HPV cobas 4800 kits. The Laboratory of Retrovirology in the discipline of Infectology at UNIFESP-EPM provided us with the CT and NG Abbott RealTime tests.
References
You might also be interested in:
2019 - Prevalence of unmet supportive care needs among Indigenous cancer patients across Australia
2012 - Prevalence of low back pain among peasant farmers in a rural community in South South Nigeria
2009 - Childhood obesity and elevated blood pressure in a rural population of northern Greece


