Introduction
Every year, despite the availability of preventive medication, tuberculosis (TB) kills millions of people, particularly in low- and middle-income countries (LMICs). In 2019, 10 million people contracted TB globally, of which 1.4 million people died1. It is well established that the reduction of active TB cases depends on effective prevention and management. As more than 80% of TB deaths and infections worldwide occur in LMICs, it is critical that prompt diagnosis and treatment in these countries are managed effectively if a significant reduction in active cases is to be achieved. Although the TB mortality rate has declined by 42% in recent decades, it stubbornly remains a leading global public health threat2. More effort is required to drive strategies toward accomplishing the global milestone of eradicating TB.
Anyone in TB-endemic countries is at risk of contracting TB, but certain populations have an increased risk of TB infection and advancing to TB disease3. These vulnerable populations include people living with HIV/AIDS, health professionals and those living in poverty. According to 2019 global data, about 208 000 people with HIV died from TB, a reduction from 678 000 in 20001. Among all those that were infected with active TB, 8.2% were individuals with positive HIV1. Further, the magnitude of TB among healthcare workers (HCWs) in healthcare settings remains higher than in the general population. The pooled incidence of active TB among HCWs was 97 per 100 000 people per year compared with the general population4. In 2019, a total of 22 314 HCWs were reported to have TB in 76 countries, with India contributing the most, accounting for 47% of the total cases, followed by China with 18%5. The WHO highlighted the positioning of healthcare facilities as key transmission sites in resource-constrained healthcare settings2. Besides this, poverty has been demonstrated to be a major determinant of TB, increasing transmission through (i) influence on living standards, such as individuals residing in poorly ventilated and overcrowded homes; (ii) delay in diagnosis and treatment; and (iii) increased susceptibility because of malnutrition6. Despite some inconsistencies, the majority of the studies have affirmed this positive relationship between individual poverty and TB in countries like South Africa, Brazil, Vietnam, Zambia and India6
In 2015, the WHO introduced the End TB Strategy to combat the global TB epidemic. Its stated aim is to decrease the TB incidence rate by 90% and reduction of TB deaths by 95% by 20357. The strategy stresses the need for prevention across all approaches, including infection prevention and control in healthcare services. These recommendations emerged because of the recurrence of TB associated with diverse factors including the upsurge in HIV infections, disruptions of access to healthcare services in LMICs due to poor healthcare systems, the emergence of drug-resistant TB and increasing incidence of non-communicable diseases (NCDs)8. According to Magee et al9, the rapid increase of the NCDs epidemic has threatened TB control in LMICs, with poor TB prevention and treatment outcomes. The scale-up of interventions to decrease the TB problem will be complicated by the complex relationship between TB and NCDs and the competition for resources between TB and NCDs in resource-limited settings9.
TB infection and prevention control strategies that were introduced by WHO and the US Centers for Disease Control and Prevention (CDC) in 1999 are widely adopted by healthcare centers to control TB transmission, including administrative, environmental and respiratory controls10. The specific strategies for each category reported to effectively reduce TB transmission in countries with high TB burden include administrative controls (triaging people with TB signs and symptoms, respiratory separation or isolation of people with assumed infectious TB, active screening, starting early effective TB treatment for people with TB disease, respiratory hygiene and cough officer), environmental controls (ventilation systems including natural, monitoring windows and hospital doors, mixed-mode ventilation (both natural and mechanical), mechanical ventilation (wall fan)) and respiratory controls (face mask, particulate respirator)10.
Although the implementation of TBIC measures has proven to reduce the risk of mycobacterium TB transmission, implementation is often limited in resource-constrained primary healthcare settings10. This systematic review of the literature aims to review the current evidence on TBIC strategies and identify the barriers and facilitators of TBIC implementation in resource-constrained primary healthcare settings.
Methods
The study protocol was registered with PROSPERO International Prospective Register of Systematic Reviews (registration number CRD42020203468). The Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines were used to develop the method for this systematic literature review (Appendix I)11.
Search strategy
Peer-reviewed articles were sourced through five online databases: ProQuest Central, ScienceDirect, Scopus, PubMed and Embase. A systematic search was conducted using keywords with Boolean operators and terms specific to the database vocabulary including tuberculosis OR TB AND infection control OR disinfect* OR quarantine* OR infection prevention OR prevent* infection AND resource-poor countr* OR developing countr* OR low-income countr* OR lower middle-income countr* AND effectiveness OR effect OR impact AND health care cent* OR health facilities OR health settings OR hospitals. Since TB infection control guidelines were first introduced in 1999, articles published after 2000 were retrieved for analysis. Although there are some potential publications before 1999, an article published after 1999 was considered appropriate as the cut-off point for examples of contemporary research relating to TB infection prevention and control measures. It has been over two decades since the publication of the WHO guideline in 1999, so this was considered sufficient to capture the most relevant work. The search was conducted from November 2020 until July 2021.
Eligibility criteria and study inclusion
The purpose of this review is to evaluate the evidence of TBIC strategies at reducing TB transmission and factors affecting its implementation at resource-poor healthcare institutions. The term ‘resource-poor health institution’ refers to ‘a locale where the capability to provide care for life-threatening illness is limited to basic critical care resources, including oxygen and trained staff. It may be stratified by categories: No resources, limited resources, and limited resources with possible referral to higher care capability’12. Health settings in LMICs are commonly characterized by limited staffing, poor infrastructures, shortages of medical supplies and drugs, and underfunding13. Therefore, ‘resource-poor settings’ in this review refers to settings in LMICs based on the World Bank data14. The terms ‘LMICs’ and/or ‘resource-constrained health settings’ or ‘resource-poor healthcare settings’ or ‘resource-limited healthcare settings’ are used interchangeably throughout this review. Furthermore, we have not included any resource-constrained setting that might exist in high-income countries. Our search strategy was limited to LMICs. This review includes peer-reviewed research published in English, conducted in resource-poor healthcare settings, and reported data on TBIC measures. For this review, peer-reviewed articles that assessed the effectiveness of some form of TBIC at primary health facilities in LMICs were selected. Table 1 provides a summary of the Population, Intervention, Comparison, Outcome, Study Design and Setting (PICOS) framework15 used to determine the eligibility criteria and screening protocol.
Table 1: Study inclusion and exclusion criteria using the Population, Intervention, Comparison, Outcome, Study Design and Setting approach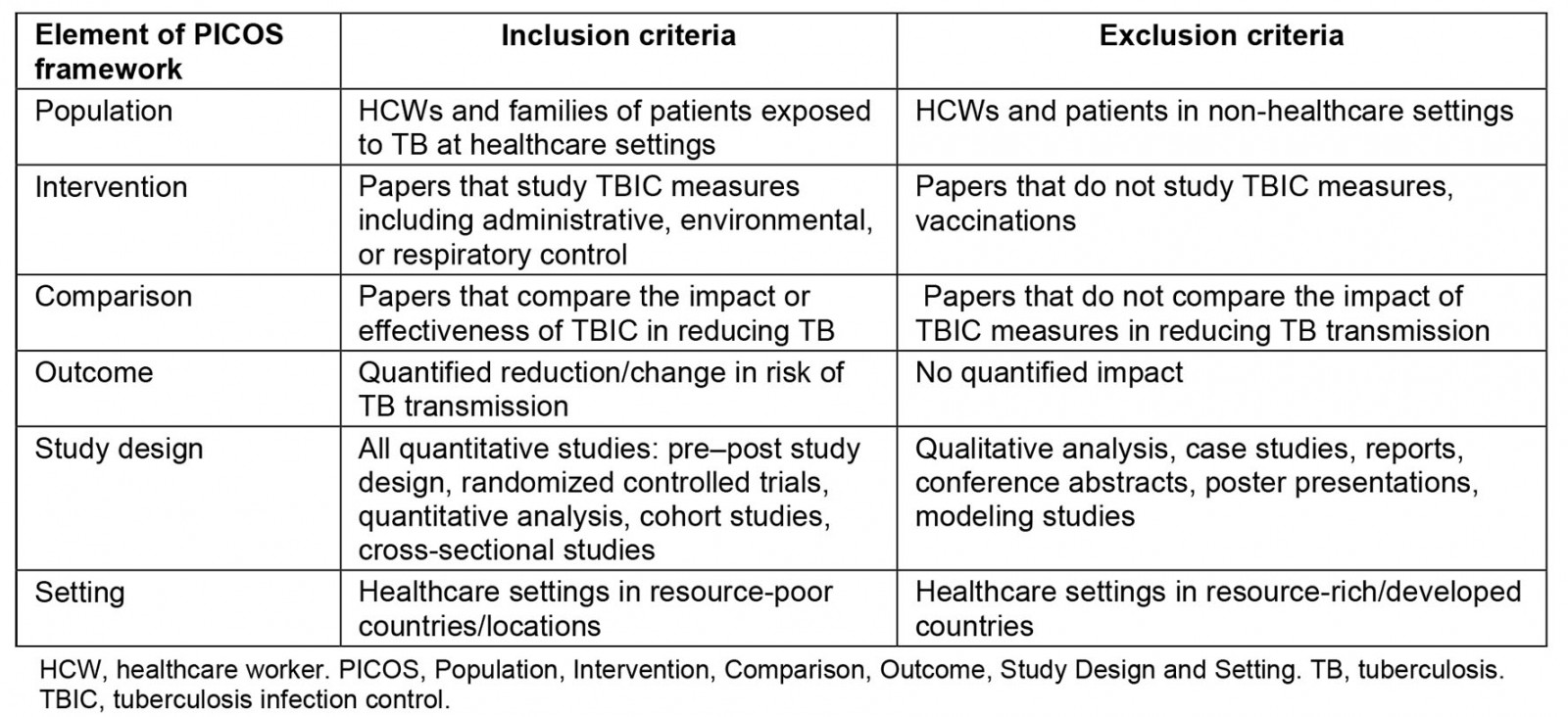
Study selection
Figure 1 presents the study selection process following the PRISMA approach of four stages16: identification, screening, eligibility and inclusion. After eliminating duplicates, the first author (GM) screened papers by reviewing the titles and abstracts against the review topic and eligibility criteria. To determine eligibility, the first author (GM) undertook a full paper review with two reviewers (SR and NH) confirming selections. The consensus was reached for the final included list of 15 papers through group deliberation.
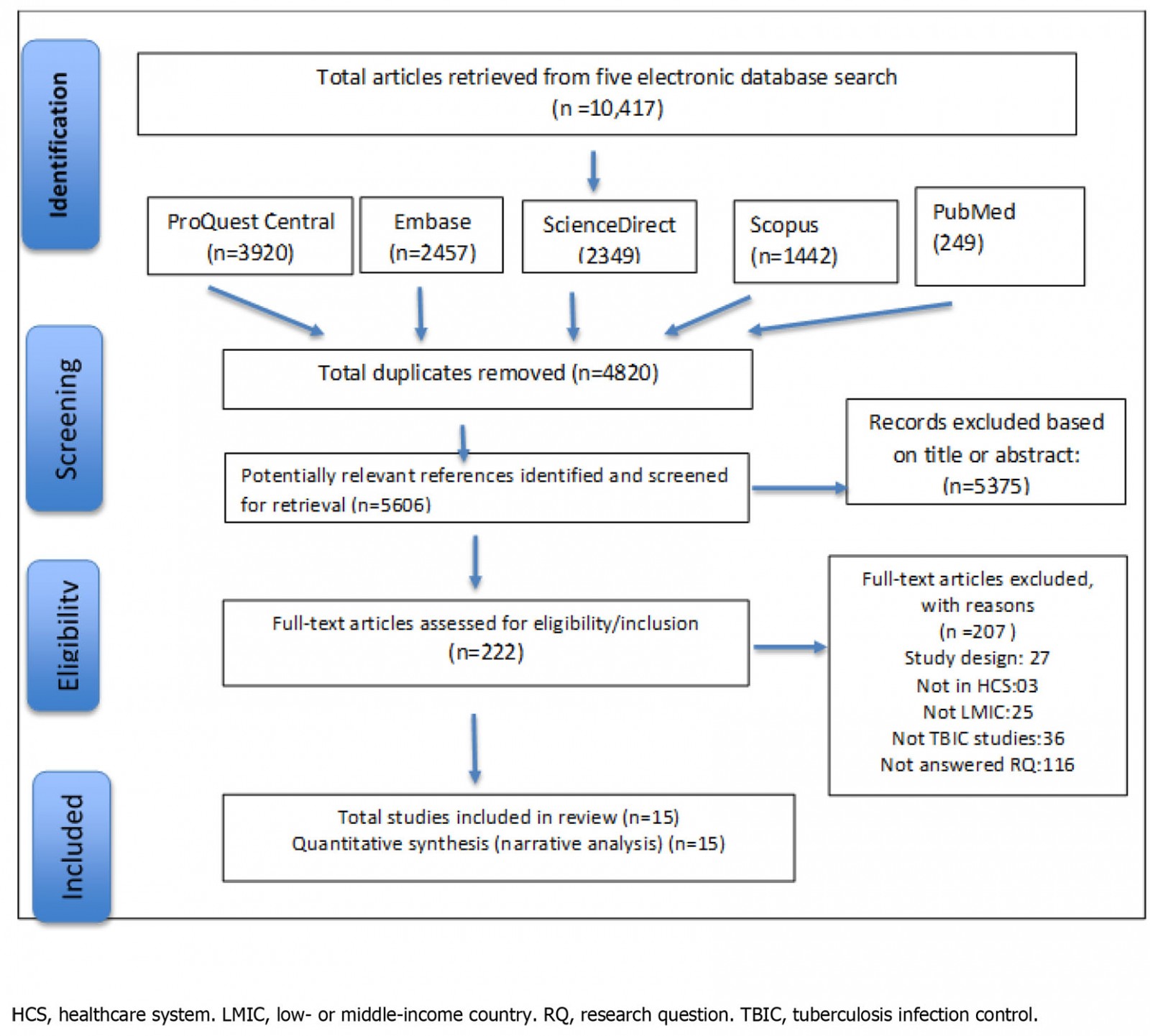 Figure 1: PRISMA flow diagram of the search strategy
Figure 1: PRISMA flow diagram of the search strategy
Data extraction and analysis
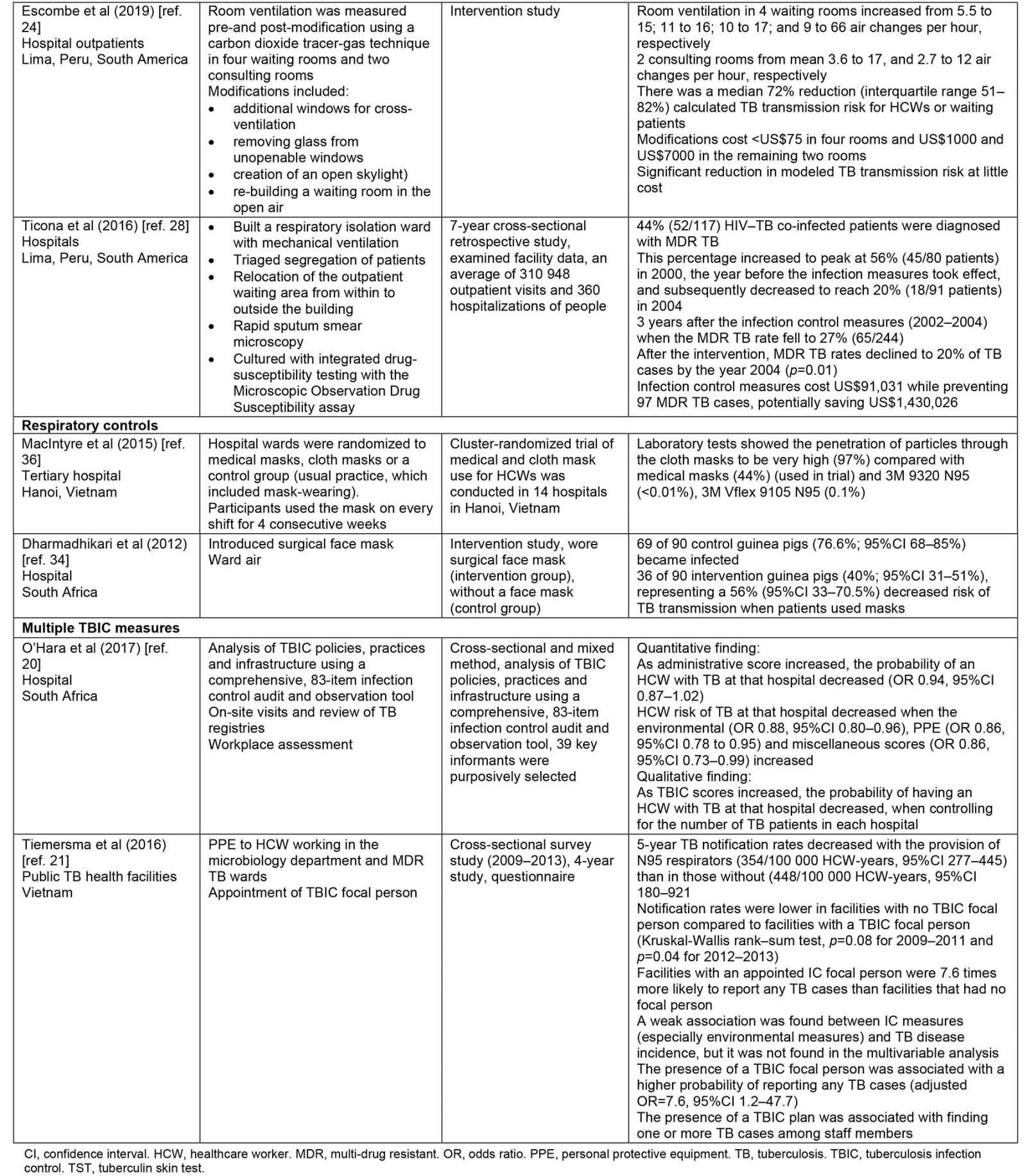
Thematic synthesis was used for data analysis. The first author (GM) independently extracted data from the selected papers that matched the study objective using a predetermined data extraction form sourced from The Cochrane Collaboration17, which was checked by two reviewers (SR and NH). The key data extracted include year, author, type of health facility, LMICs, interventions (such as administrative control, environmental control, respiratory controls), study design and main outcomes. This information is summarized in Table 2.
Table 2: Summary of included studies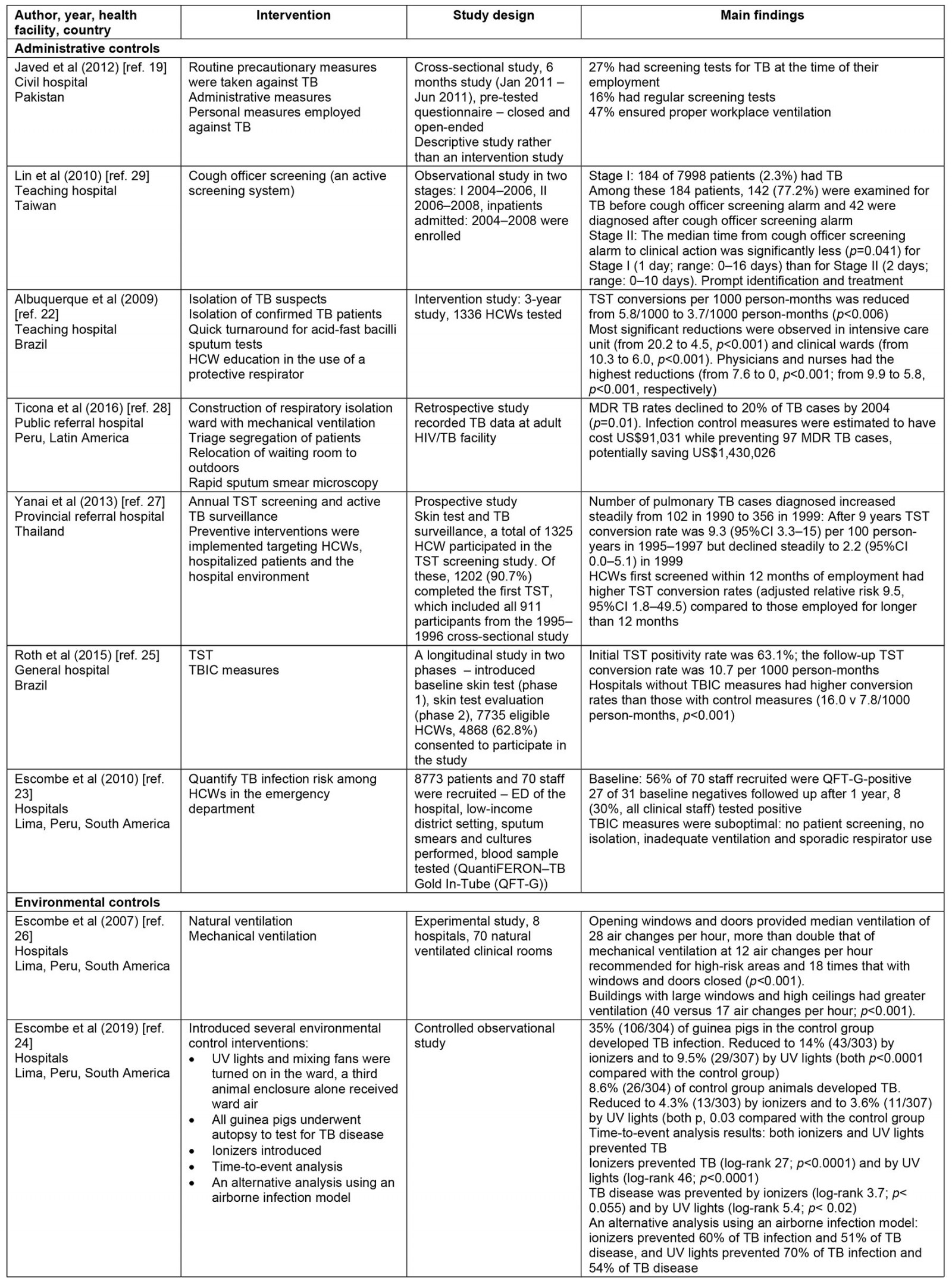
Quality assessment (risk of bias)
The authors conducted a quality assessment during the data extraction process. Each study underwent quality assessment using the National Institute of Health (NIH) study assessment tool18. The NIH represents a tool that assesses scientific rigor and is frequently used by researchers to assess studies in public health interventions. The tool includes 10 criteria that evaluate the relevance to practice and the scientific validity of each study. Employing this tool, three independent reviewers assessed the quality of the studies and rated each article as good, fair or poor. All 15 studies in the final list were deemed eligible for the comprehensive review and subject to data extraction.
Ethics approval
Ethics approval was not required for this systematic review, as it uses publicly accessible documents as evidence and does not collect personal, sensitive, or confidential information from participants.
Results
A total of 10 417 studies were retrieved from the five online databases. Of those studies, 222 studies were selected for full-text review, 15 of which met the eligibility criteria and were included for data extraction. More than half (5375) of the studies were excluded because they were either not relevant to the topic, were not focused on TBIC measures, or were not original research papers.
Summary of included studies
A summary of the characteristics of the included studies is presented in Table 2. The selected studies were conducted in both private and state-owned hospitals and TB primary healthcare settings across four WHO regional groupings including the Regions of the Americas, South African Region, South-East Asia Region and Regions of the Mediterranean. None of the studies were conducted in rural health services. Most were conducted in the Regions of the Americas. The 15 studies comprised four cross-sectional studies19-21, four intervention studies22-25, one experimental study26, one prospective study27, two retrospective studies25,28, two observational studies29,30 and one randomized controlled trial study31. These studies have demonstrated consistent although not extensive evidence of TBIC measures in reducing TB transmission. The major themes have been organized according to WHO guidelines for TBIC in healthcare settings including administrative control, environmental control and respiratory control.
Administrative controls
Administrative controls consist of practices to reduce exposure and thus minimize direct transmission of active Mycobacterium TB(2}. Of the 15 included studies, five studies assessed the effectiveness of administrative controls in protecting healthcare workers (HCWs) and patients against active TB19,22,25,27,29.
Of the five studies, four identified a statistically significant relationship between administrative controls and a gradual reduction in TB transmission. The specific administrative measures identified in these studies include a cough officer screening system, patient isolation, triage, a rapid turnaround for sputum test result (usually test results are available within 24 hours), HCW education on the proper use of protective respirators, effective tuberculin skin testing (TST), active TB screening and surveillance to detect active TB early to initiate prompt treatment and rapid diagnosis, and effective treatment22,25,27,29.
The four studies indicated that the implementation of these measures provides a protective effect for HCWs. A longitudinal study from Brazil has shown that TST, rapid diagnosis and treatment, and patient isolation lead to lower conversion rates among HCWs25. Brazilian hospitals without the implementation of administrative TBIC strategies have a higher conversion rate compared with hospitals with infection control measures (16.0 v 7.8/1000 person-months, p<0.001). Furthermore, a retrospective study found that multi-drug resistant TB (MDR TB) rates declined by 20% (p=0.001) after introducing isolation wards and patient triage28. In this setting, these preventive measures were estimated to have cost US$91,031 (~A$130,700) while preventing 97 MDR TB cases, potentially saving US$1,430,026 (~A$2,054,000)28.
The fifth study on administrative control measures is a 3-year intervention study that focused on the effect of TST infection control measures on TB reduction22. This study found utilizing monthly TB skin tests, isolation of TB suspects and confirmed TB patients, quick turnaround for acid-fast bacilli sputum tests and health worker education in the use of protective respirators for 1000 health workers employed in the intensive care unit and clinical wards has resulted in a significant reduction in TB cases from 20.2 to 4.5 (p<0.001) and from 10.3 to 6.0 (p<0.001), respectively22. The study authors stressed that the infection control interventions cannot be sustained without support from local and national health managers. Thus, strong leadership in the health system is crucial for successful implementation of TBIC measures22.
This review demonstrates that a combination of multiple administrative control measures like patient isolation, the rapid turnaround for sputum tests and HCW education are more successful than a single method22. Our results suggest that simple administrative control measures, which are inexpensive and easy to implement in resource-constrained primary healthcare settings, can be efficient in reducing TB.
Environmental controls
Of the 15 included studies, four studies evaluated the effectiveness of environmental control in reducing TB in healthcare facilities24,26,30,32. The environmental controls represent specific measures to reduce the high concentration of infectious bacteria in the air, thereby reducing the danger of TB transmission2. This preventive measure is accomplished through the introduction of a ventilation system to improve air circulation to disinfect the air2.
Of the four studies, three studies utilized natural ventilation systems by opening windows and doors in healthcare facilities24,26,30,32. The other study measured implementation of minimal low-cost modifications to existing hospital waiting and consultation rooms and air circulation systems26. All four studies found that, after the direct interventions, there was a significant improvement in air circulation. One study found that, after modification of windows and construction of an outdoor waiting room in four separate rooms at the outpatient waiting rooms, air circulation dramatically increased, from 6 to 70 air changes per hour26. The other study that measured the effect of direct alteration to current hospital waiting and consultation rooms found similar air changes per hour, preventing the spread of TB among HCWs and waiting patients24. Consequently, there was a median 72% reduction in possible TB transmission risk for health workers and waiting patients24. The modifications to the existing hospital infrastructure cost US$8000 (~A$11,500). Thus, minor changes to existing physical infrastructure in the hospital have considerably increased natural ventilation and therefore significantly reduced possible TB transmission at little cost24.
Respiratory protection
Two of the 15 papers included in the review assessed the effectiveness of respiratory protection33. Respiratory protection controls aim to reduce the risk of potential exposure to active TB and other respiratory diseases for local health workers employed in risky health care environments2.
The two studies both assessed the efficacy of respiratory face masks on TB prevention in hospitals33,34. Both studies found a positive relationship between wearing respiratory face masks and TB prevention. The first study was a randomized control trial from Hanoi, Vietnam, which examined the efficacy of medical and cloth masks and found that cloth masks resulted in significantly higher rates of infection than medical masks35. Laboratory examinations showed higher penetration of particles through the cloth masks (97%) compared with the medical masks (44%). Further, the efficacy of medical masks in this study translates to 92% protection against respiratory infections, suggesting a reduced risk of infection with medical masks. Consequently, cloth masks are not recommended for health workers, particularly in high-risk settings35. A recent randomization trial showed that cloth masks were inferior to medical masks and really exposed the wearer to the risk of nosocomial infection36.
The second study measured the efficacy of surgical face masks when worn by patients with MDR TB33. Over 3 months, 17 patients with pulmonary MDR TB occupied an MDR TB ward in South Africa wore face masks on alternate days. Ward air was exhausted to two identical chambers, each accommodating 90 pathogen-free guinea pigs that breathed ward air either when patients wore surgical masks (intervention group) or when patients did not wear masks (control group). The results showed that 90 controlled guinea pigs contracted TB, compared with 36 of 90 intervention guinea pigs, demonstrating a 56% reduced risk of TB transmission when patients used masks33. This study demonstrates that the use of face masks on infectious TB patients can significantly reduce TB transmission and offer protective measures against TB infection.
Combined TB infection control measures
Two of the 15 papers in this review reported on the implementation of a combination of administrative, environmental and respiratory control measures20,21. The two papers from South Africa and Vietnam conducted in public hospitals reported on the implementation of strategies from all three categories and found that personal protective equipment (PPE), N95 respirators and employment of a TBIC focal person were highly effective in reducing TB transmission. The study that examined the effect of N95 respirators used for health personnel employed in a microbiology department and MDR TB ward found a significant reduction in TB notifications21. For instance, 5-year TB notification rates decrease with the provision of N95 respirators (354/100 000 HCW-years, 95% confidence interval (CI) 277–445) compared with those without N95 respirators (448/100 000 HCW-years, 95%CI 180–921)20. Additionally, the appointment of a TB focal person was associated with adequate reporting of active TB cases among HCWs. TB notification rates were lower in health facilities with no TBIC focal person compared with facilities with a TBIC focal person (Kruskal-Wallis rank–sum test p-values of p=0.08 for 2009–2011 and p=0.04 for 2012–2013)20. Furthermore, health facilities with an infection control officer were 7.6 times more likely to report any TB cases than facilities that had no infection control person20. This suggests that the probability of being infected with TB decreases as infection control measures increase.
Barriers and facilitators associated with implementation of TB infection control measures
This review found several barriers that impeded and/or facilitators that mediated the effectiveness of infection control measures at resource-limited healthcare settings. Administrative controls allow prompt identification, social isolation and diagnosis, which decreases the infection of air due to TB2. The lack of patient education and unsupportive workplace culture have negatively affected the implementation of these administrative control measures20. Further, the availability and appropriate use of PPE protect HCWs from contracting TB. However, inadequate supply of particulate respirators has affected the effective implementation of respiratory control measures20, while insufficient isolation facilities and physical infrastructure limitations have obstructed the effective implementation of environmental control practices at health facilities20,27,29. The finding that these barriers may impinge on the implementation of TBIC measures is supported by earlier studies conducted in China37 and Uganda38.
Facilitators that were reported to strengthen the implementation of TBIC measures pointed towards the establishment of a strong healthcare system for the successful implementation of health policies. Triaging patients with suspected TB, maintenance and refurbishment of health infrastructure were identified as facilitators to administrative control20. Additionally, appropriate use of PPE, training HCWs on the correct use of PPE, positive influence of infection control champions (eg cough officers) at the healthcare settings and continuous HCW educational training on prevention strategies were reported as facilitators to implementation of TBIC measures at healthcare facilities22,27,29. Generally, strengthening the capacity of the healthcare system at the grassroots level through health workers’ knowledge and education in the provision of healthcare services is also reported by Alotaibi and colleagues39 in Saudi Arabia.
Discussion
The conclusions were drawn from the diverse study designs found in this research. Our review found consistent evidence of the benefits of implementing WHO-supported strategies on TBIC measures over the decades in reducing TB transmission among HCWs, patients and the community in LMICs. This discussion is structured around the three recognized categories of intervention for TBIC (administrative, environmental and respiratory controls) and highlights the strategies that have been evidenced as most effective for TBIC in healthcare facilities in LMICs.
Administrative controls are regarded by WHO as the first and most important level of the TBIC hierarchy2. These are management strategies that are meant to decrease the threat of exposure to people with infectious TB40. This systematic review identified cough officer screening systems, rapid diagnosis and treatment, isolation and triaging of cough patients, tuberculin skin testing and active screening as effective administrative control measures. Collectively, these control measures are found to effectively reduce TB transmission among HCWs and patients. However, of particular note was the reported success of cough officer interventions introduced in a hospital29. This intervention represents a relatively low-cost initiative that mobilizes a designated health worker(s) within the organization to take action29. By designating a nursing staff member from the general ward as a cough officer, the individual takes responsibility to prioritize action when necessary. All cough officers undergo training on infectious disease control, questioning technique, recording cough conditions and entering data into the computerized cough officer screening system. This positioning has also been effectively used to assist in the control of drug-susceptible and drug-resistant TB in KwaZulu-Natal Province, South Africa41. At a conceptual level, the cough officer intervention aligns with the strategy of active case finding42. This involves the early detection of active TB among individuals who present to healthcare services with symptoms indicative of TB. Early diagnosis and effective treatment of TB cases are crucial to not only reduce diseases and deaths but also decrease TB transmission within the community42,43. Hence, the cough officer screening system was introduced within a hospital to identify patients with pulmonary TB early and to reduce its transmission within the hospital to enhance passive case finding29. This measure is cost effective and can be easily applied in resource-constrained healthcare institutions in LMICs.
Triaging (prioritization of patients who have a cough for more than 2 weeks) and separation of presumptive TB patients from other patients in health settings were identified as important administrative control measures. While agreeing that these measures are based on scientifically proven strategies, the concern is being able to translate such strategies into practice in low-income, high TB burden countries where health facilities are often small and overcrowded with inadequate space for triage and isolation44. Future TBIC guidelines could consider an alternative control measure to prevent TB transmission in resource-poor settings to address implementation gaps. To address this implementation gap, a recent study suggests that health facilities should consider the verandas and corridors as isolation space36. A similar arrangement was formalized in Malawi by arranging isolation areas outdoors when indoor waiting areas were overcrowded45. If the healthcare facilities use verandas and corridors as isolation space to manage overcrowding, then patients remain at the health facility and are given proper TB treatment. Therefore, TB transmission among the HCWs and community is prevented.
Environmental control measures decrease the concentration of airborne infectious bacteria in the air46. Among environmental controls, the introduction of ventilation systems remains a priority because ventilation decreases the number of infectious bacteria in the air46. This review found keeping doors and windows open and making minor changes to existing waiting and consultation rooms in hospital settings have significantly improved ventilation systems26,30. An experimental study in Peru showed that natural ventilation generated more than 17–40 air circulations per hour, while well-established mechanical ventilation in isolation rooms generated 12 air circulations per hour26,46 Further, buildings with large windows and higher ceilings had higher ventilation compared to small windows and low ceilings26. This arrangement does not require increased resources and can be easily implemented in resource-constrained healthcare facilities. While natural ventilation is cost-effective its acceptance depends on the local climate and may not be appropriate due to cold climate, mosquitoes and security reasons46. However, when used alone, increasing ventilation is not enough to protect individuals from exposure to the pathogens that cause TB. WHO stressed that natural ventilation should be used along with other recommended practices such as physical distancing, and avoidance of crowded indoor spaces, as well as wearing masks and hand-washing40.
Another intervention study from Lima, Peru measured the impact of minor modifications in waiting and consultation rooms and air circulation in a hospital24. The minor modifications included repair and construction of new windows in the sidewall and construction of a separate outdoor waiting room, particularly for respiratory outpatients in waiting and consultation rooms24. After the interventions, there was a significant improvement in room ventilation in the four waiting rooms, thus preventing TB transmission. As a medium to long-term strategy, new or renovated facilities should make appropriate ventilation a high priority47.
Respiratory protection control, the third level of the hierarchy, is the use of respiratory protection design to reduce the risk of exposure to active TB for health personnel in high-risk settings29. An experimental study showed that patients who had face masks were protected from contracting TB compared with patients who had no face mask33. This study highlighted the importance of using face masks, particularly when meeting infectious patients. A meta-analysis conducted in Canada affirms results presented in this review regarding the usefulness of wearing face masks48. Despite its significance, face masks were not always applied despite availability at the health facility36. A recent study shows that although adequate masks were provided at the health facility, they were not worn by HCWs in some healthcare settings despite increasing exposure to infectious patients in Nigeria49. This trend may increase opportunities for TB transmission and other respiratory infections in healthcare settings, suggesting that the adoption of TBIC measures is required in healthcare facilities delivering TB healthcare services in LMICs. Of concern, cloth masks are commonly used in LMICs to prevent the spread of TB from patients. However, a recent randomized controlled trial showed that cloth masks are inferior to medical masks and are not recommended for use, particularly in high-risk settings36. While there are limited studies, cloth masks should be discouraged to protect the wearers from new infections.
Strengths and limitations
The major strength of this systematic review is that it synthesized the current evidence on the implementation of TB control strategies at the health facility level in low-income, high TB burden countries. Despite this key strength, there are several limitations of this research. The primary limitation represents the apparent lack of high-quality published papers using randomized control trials that measured the effectiveness of effective TBIC interventions to help support best infection control practices in LMICs. Another limitation of the review may be in the language bias, as only publications in English were included, which excluded pertinent studies in other local languages. Despite our collaborative efforts to retrieve all relevant articles, it is likely that not all relevant studies are included in this review. Some valuable information could be missing, especially conference papers and poster presentations. Due to the limited number of studies in this review, generalizability is problematic. Finally, there may be some resource-poor health facilities in high-income countries, but these were beyond the scope of our study.
Implications for policy and practice in resource-limited primary healthcare settings
The high burden of active TB identified in LMICs emphasizes the need for an effective public health strategy to improve this complex situation. The WHO recommends that TBIC measures identified in this review can reduce TB transmission in high TB burden locations2. One outstanding example would be the implementation of the cough officer screening system in hospitals29. This strategy maintains an active screening system in hospital inpatients to improve TB detection rate among the inpatients. This system has improved the detection of TB by reducing the delay from infection to diagnosis, therefore preventing TB transmission among other patients29. A critical aspect of effective implementation of the cough officer screening system would be the need to continue training for nurses on TB diagnosis and infection control guidelines.
Another example of TBIC measures is the use of natural ventilation systems like opening doors and windows in TB wards in healthcare facilities24. This approach can supplement the administrative control measures to reduce exposure to TB transmission by improving air circulation as demonstrated in several studies24,26,32. An important aspect of the implementation of this strategy is training for health workers on good ventilation practices. Maintenance and refurbishment of health infrastructures such as the patient consultation rooms and inpatient wards would be equally crucial, particularly in the context of overcrowded settings in health centers, although these actions would be more costly and need more medium-term strategies and planning.
The use of respiratory masks has the additional benefit of contributing to TB reduction. In particular, this strategy reduces the risk of exposure to Mycobacterium TB for health workers in specific areas and circumstances2. An essential component of the use of respiratory masks would be the need for health worker training on the proper use of PPE and a steady supply of respirators. It is therefore equally important to consider whether masks are economically and logistically feasible interventions in settings with a high burden of TB.
As a short-term strategy, effective implementation of TBIC measures in resource-poor healthcare settings would require the improvement of the current health facilities, particularly the TB wards, outpatients waiting and consultation rooms. In the long term, investment in healthcare systems such as infrastructure designed for infection control, medical supplies, health financing, healthcare workforce, and governance and leadership will be important for the effective implementation of TBIC measures.
Conclusion
This review demonstrated that the implementation of TBIC measures including administrative, environmental and respiratory control measures have prevented TB transmission in resource-limited settings. Simple and low-cost interventions such as a cough officer screening system, patient isolation and triaging, minor modifications to infrastructure, opening windows and doors and HCWs’ utilization of respiratory masks are effective. Collectively, these measures are highly effective in reducing TB transmission and can be easily adopted in health facilities with limited resources accompanied by the right supportive mechanisms such as patient education, supportive workplace culture, availability of PPE, adequate supply of respirators and adequate isolation facilities. Health institutions in locations where TB remains high in communities must invest in improved implementation of these measures to protect their HCWs and to reduce the community burden of TB disease. While TBIC strategies have the potential to reduce active TB transmission, if they are not correctly implemented in resource-constrained settings it will be difficult to achieve the global aim of the WHO End TB Strategy.
Funding
The authors declare that there is no financial support given towards this research. The first author is supported through university tuition and living allowance scholarships.
Acknowledgements
The principal author, Gigil Marme, would like to acknowledge Griffith University for the PhD scholarship.
References
appendix I:
Appendix I: Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) checklist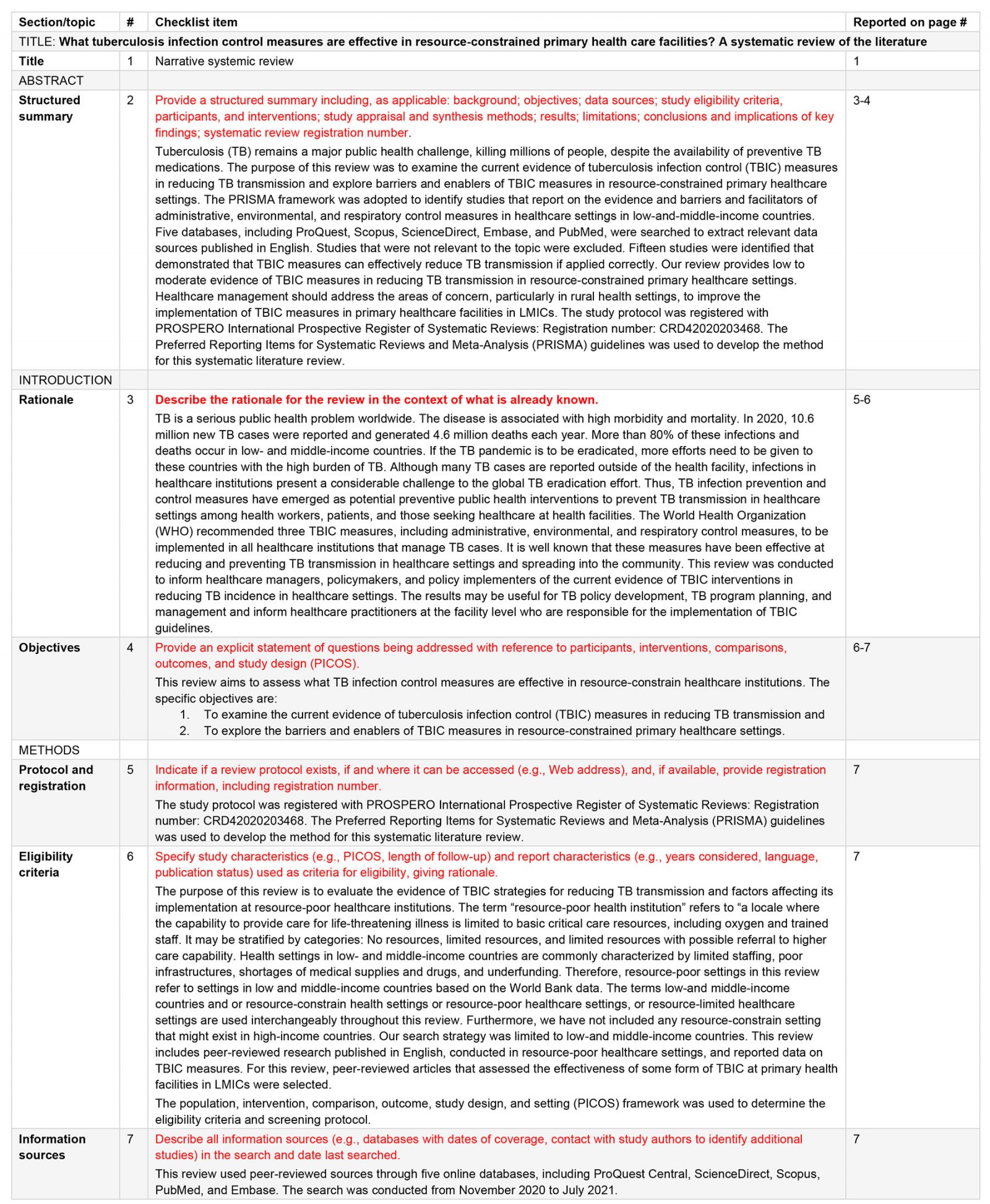
You might also be interested in:
2022 - ‘Inclusive’ health systems increase healthy life expectancy
2016 - Contribution of military psychology in supporting those in rural and remote work environments



