Dear Editor
The Mediterranean dietary pattern is a well-established protective factor against cardiovascular disease (CVD)1. However, very often it is not sufficient and, in the treatment of dyslipidaemia in patients with high cardiovascular risk, we often need to add 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors (statins)2. Liver toxicity is a known but rare side effect of statins3, and cross-reactivity among the different statins in the susceptibility to liver injury has not been defined. Here we report a case of atorvastatin-induced hepatitis, partially relapsed after rosuvastatin assumption, in a patient tolerant to simvastatin.
Consent to publish this case was obtained from the patient. Confidentiality and anonymity were assured. The study was conducted in accordance with the Declaration of Helsinki.
A Caucasian woman aged 72 years, affected by high blood pressure, dyslipidaemia, subclinical hypothyroidism and osteoarthritis, was treated for several years with simvastatin 40 mg qd. Because of an episode of atrial fibrillation (AF), treated with ablation in 2016, she had also initiated therapy with warfarin and flecainide 100 mg bid.
In September 2019 the patient was hospitalised for AF recurrence, complicated by acute myocardial infarction, and she was treated with angioplasty and coronary stenting. At discharge she was prescribed apixaban 5 mg bid, acetylsalicylic acid 100 mg qd, lansoprazole 15 mg qd, clopidogrel 75 mg qd, atorvastatin 40 mg qd, olmesartan 40 mg qd, bisoprolol 2.5 mg qd and amlodipine 5 mg qd.
Several weeks later the patient started complaining of worsening generalised itching, then skin jaundice appeared, and in October 2019 she was again admitted to the hospital. Laboratory analyses revealed hepatic injury: total bilirubin (TB) 3.9 mg/dL, direct bilirubin (DB) 2 mg/dL, alanine aminotransferase (ALT) 827 U/L, aspartate aminotransferase (AST) 890 U/L, gamma-glutamyl transpeptidase (GGT) 201 U/L.
At hospital the hepatic injury was diagnosed as an adverse side effect of apixaban, which was stopped and substituted with enoxaparin. Clopidogrel and atorvastatin were also discontinued on a precautionary principle basis. Symptoms rapidly improved and at discharge TB was 2.7 mg/dL, ALT 511 U/L, AST 380 U/L and GGT 149 U/L.
A month after discharge the patient suspended enoxaparin and started warfarin. Hepatic profile progressively improved until normalisation (in January 2020 TB 1.2 mg/dL, DB 0.2 mg/dL, ALT 20 U/L, AST 24 U/L, GGT 18 U/L). In August 2020, statin therapy was recommended because of the high level of low-density lipoprotein (LDL) cholesterol (143 mg/dL): on 4 August a combination therapy of rosuvastatin 20 mg qd and ezetimibe 10 mg qd was started. Three days later the patient started to complain of itching again; after a week, blood tests showed a slight increase of bilirubin (TB 1.49, DB 0.4 mg/dL) with normal levels of transaminases and GGT. Statin therapy was immediately suspended, and cholestyramine was prescribed, with fast improvement of itching. In the same month, a therapy with ezetimibe 10 mg qd and food supplementation with monacolin K was started, without side effects. Blood tests performed three months later (November 2020) showed poor control of lipidic profile (total cholesterol 261 mg/dL, high density lipoprotein (HDL) cholesterol 59 mg/dL, triglycerides 145 mg/dL, calculated LDL cholesterol 173 mg/dL), thus simvastatin 20 mg was restarted at a dose of 20 mg/day, in association with ezetimibe. The patient is currently tolerating this treatment without adverse reactions such as itching. Blood tests performed in February 2021 show an acceptable liver profile (TB 1.26 mg/dL, DB 0.22 mg/dL, ALT 9 U/L, AST 22 U/L, GGT 18 U/L), in addition to an improved lipidic profile (total cholesterol 196 mg/dL, HDL cholesterol 55 mg/dL, triglycerides 136 mg/dL, calculated LDL cholesterol 113.8 mg/dL).
Statins are generally well tolerated, and a mild elevation of serum aminotransferases is reported in up to 3% patients within 1 year of treatment4,5. Thus, we consider our case report very rare and interesting, because the patient underwent three switches from one statin to another, showing different tolerance profiles: from simvastatin (tolerated) to atorvastatin (cholestatic hepatitis after several weeks), then to rosuvastatin (mild relapse of cholestasis after a few days), finally back to simvastatin (tolerated) (Table 1).
In our opinion this reported case confirms the concept that, regarding liver toxicity, single statins have different risk profiles. The knowledge and trust generated by the repeated contact between the patient and her general practitioner, typical of a rural setting, supported the doctor's confidence in resuming statin therapy in this problematic case.
Table 1: Statins – different levels of efficacy and liver toxicity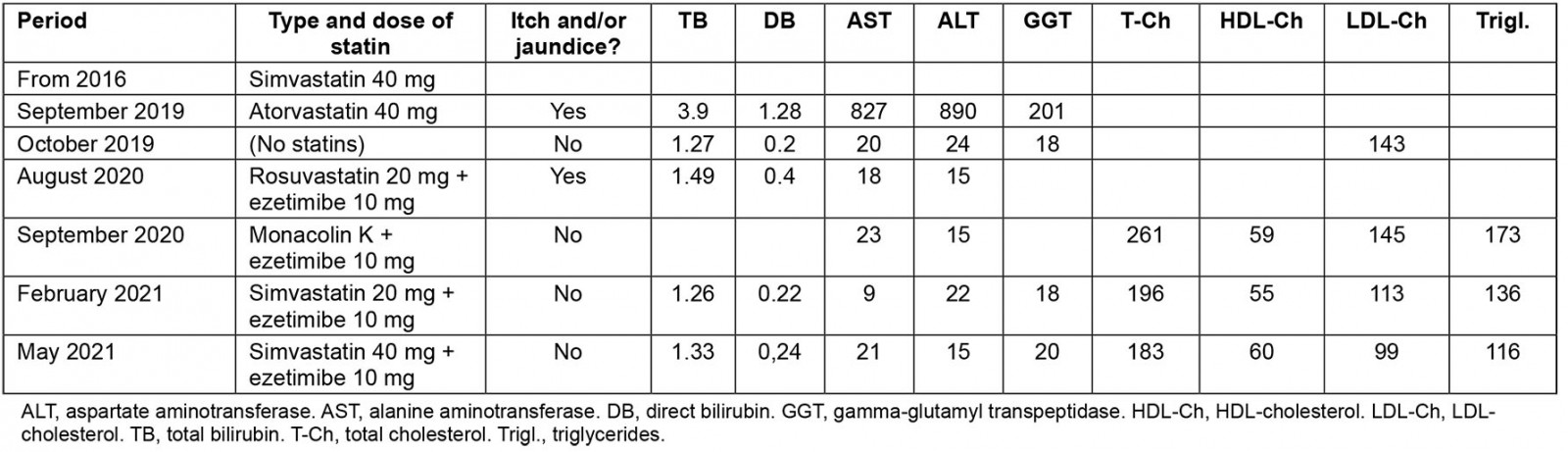
Cecilia Soavi, MD; Marcello Cavicchi, MD; Angelo Cavicchi, MD, AUSL Ferrara, Emilia Romagna Region, Italy
Ferdinando Petrazzuoli, MD, PhD, Department of Clinical Sciences in Malmö, Centre for Primary Health Care Research, Lund University, Malmö, Sweden




