Introduction
Magnesium is an essential cation in the human body and is a cofactor for more than 300 enzymatic reactions1,2. Magnesium is required for almost all body system functions, including cardiovascular, neurological, endocrine, musculoskeletal and immune systems1,3. Hypomagnesaemia is linked to many diseases, such as myocardial ischaemia, cardiac arrhythmia, epilepsy and diabetes mellitus1. The estimated prevalence of hypomagnesaemia in patients ranges between 5% and 20% in hospitalised patients in general wards4-6. Furthermore, the prevalence of hypomagnesaemia can be further increased in patients admitted to the intensive care unit (ICU) (up to 65%)7. Hypomagnesaemia has been associated with poor health outcomes in hospitalised patients, including increased length of hospital stay, inpatient mortality and need for admission to high acuity areas8. After hospital discharge, hypomagnesaemia has also been associated with increased mortality compared to patients with normomagnesaemia9,10. Hypermagnesaemia is less common than hypomagnesaemia and poorly studied among hospitalised patients. However, studies have demonstrated that hypermagnesaemia also has poor health outcomes in hospitalised patients5,11,12. A previous study evaluated hypomagnesaemia among hospitalised patients in the Northern Territory, Australia; however, the study included only patients diagnosed with hypomagnesaemia according to the International classification of diseases (10th revision) codes, only from one hospital in the region and excluded all patients with normomagnesaemia as well as hypermagnesaemia; hence assessment of the prognostic value of dysmagnesaemia was limited13.
This study aimed to assess the prevalence of dysmagnesaemia and associated health outcomes among all hospitalised patients in the Northern Territory.
Methods
Study design, setting and population
This was a retrospective, longitudinal data-linkage study conducted in the Northern Territory, of all hospital admissions between 1 January 2008 and 31 December 2017. We included admitted adult patients (≥18 years) from all publicly funded hospitals, namely the Royal Darwin Hospita, Alice Springs Hospital, Gove District Hospital, Katherine Hospital and Tenant Creek Hospital. These hospitals offer a diverse range of specialised healthcare services to a population of approximately 246 000 living in a vast area of 1.35 million km214. The region primarily comprises rural and remote communities. Of the population in Northern Territory, about 30% identifies as First Nations People14.
Data collection
We used individual patient-level data from hospital inpatient data, mortality data from the Northern Territory Department of Health administrative data universes, and hospital laboratory data. Relevant demographic data, including age, gender, First Nations status, and remoteness, were collected. In addition, we gathered relevant laboratory results data, including calcium, potassium, phosphate, haemoglobin, albumin and eGFR levels. Finally, important health outcomes were collected, including the length of hospital stay (LOS), ICU admission, ICU LOS, as well as inpatient and post-discharge all-cause mortality up to 31 December 2017.
Definitions
The primary diagnosis for admission was determined using the 10th revision of the International classification of diseases (10th revision)15. Remote areas were defined as all areas outside Northern Territory major cities as defined by the Australian Statistical Geography Standard16.
Stratification by magnesium levels
Serum magnesium concentrations were part of routine laboratory investigations for all hospitalised patients. The patients were initially stratified into five groups based on their initial serum magnesium level within 7 days of admission. Normal magnesium level was defined as between 0.70 and 1.05 mmol/L, mild hypomagnesaemia was defined as between 0.51 and 0.69 mmol/L, and moderate to severe hypomagnesaemia was defined as ≤0.5 mmol/L. In addition, mild hypermagnesaemia was defined as between 1.05 and 2.19 mmol/L, and moderate to severe hypermagnesaemia was defined as ≥2.2 mmol/L. To improve the numbers in certain calculations, the patients were then stratified into only three groups (normal magnesium level, hypomagnesaemia and hypermagnesaemia). Finally, dysmagnesaemia was defined as all patients with abnormal magnesium levels (ie hypomagnesaemia and hypermagnesaemia). Multiple admissions were considered. However, the first admission with a low magnesium was the criteria for stratification.
Statistical analysis
Categorical variables were presented as proportions and numbers, while continuous variables were reported as either mean±standard deviation (SD) for normally distributed data or median (interquartile range) for non-normally distributed data. To compare continuous variables among the five groups, the Kruskal–Wallis rank sum test was employed. In addition, c2 test or Fisher’s exact tests were utilised to evaluate the relationship between categorical variables. To analyse the mortality outcome, the Kaplan–Meier method and log-rank tests were used for comparisons. The hazard ratio and 95% confidence intervals (CIs) were computed. Two-sided p-values <0.05 were deemed statistically significant. Analysis was conducted using R (R Core Team, 2022) (R Foundation for Statistical Computing, Vienna, Austria).
Ethics approval
The study was approved by the Human Research Ethics Committee of the Northern Territory Department of Health and Menzies School of Health Research (HREC-2015-2501).
Results
A total of 22 293 patients were admitted during the study period, with a median follow-up period of 5 years. All patients had a measured magnesium level. Among them, 392 patients (1.76%) had moderate to severe hypomagnesaemia, and 6212 patients (27.87%) had mild hypomagnesaemia. Thus, 6604 patients (29.62%) had hypomagnesaemia. Only nine patients (0.04%) were found to have moderate to severe hypermagnesaemia, while 466 patients (2.09%) were found to have mild hypermagnesaemia. Accordingly, 475 patients (2.13%) had hypermagnesaemia. Therefore, the prevalence of dysmagnesaemia among hospitalised patients was 31.75% (95%CI: 31.15–32.35) (Table 1).
More females than males presented with moderate to severe hypomagnesaemia (50%) and moderate to severe hypermagnesaemia (56%). Those with moderate to severe hypermagnesaemia were older than the other four groups of magnesium. Moreover, patients with severe hypomagnesaemia and severe hypermagnesaemia had lower mean calcium levels (p<0.001). In addition, more First Nations patients (60%) and patients with lower estimated glomerular filtration rate (eGFR) values (p<0.001) were present in the moderate to severe hypomagnesaemia group compared to the other four groups of magnesium (p<0.001). Hypomagnesaemia was very prevalent (n=3278/7600; 43.13%) among the hospitalised Australian First Nations populations. The normomagnesaemia group had higher mean values of haemoglobin (129±21 g/L; p<0.001) and albumin level (37±5 g/L; p<0.001) (Table 1).
As shown in Table 2, as a principal diagnosis certain infectious and parasitic diseases (13%), genito-urinary disease (8.3%), diseases of the musculoskeletal system and connective tissue (3.2%), and diseases of endocrinology, nutrition and metabolism (7.7%) were more prevalent in those with severe hypomagnesaemia. Although respiratory system diseases as a principal diagnosis were prevalent in those with severe hypermagnesaemia (33%), it was also prevalent in those with severe hypomagnesaemia (17%). Additionally, cardiovascular disease were more prevalent as a principal diagnosis in those with severe hypermagnesaemia (33%).
Table 3 demonstrates the clinical outcomes associated with dysmagnesaemia. All levels of hypomagnesaemia were associated with a longer LOS (p<0.001). Also, all levels of hypermagnesaemia were associated with a longer LOS in ICU (p<0.001). In addition, less mortality (13%, 95%CI: 13–14%) was observed in the normal magnesium group than in the dysmagnesaemia groups (p<0.001).
Figure 1 depicts the survival analysis stratified according to the three groups of serum magnesium levels (hypomagnesaemia, normomagnesaemia, and hypermagnesaemia). Overall, patients hospitalised with dysmagnesaemia had an increased mortality rate during the study period than those with normal magnesium levels. Furthermore, patients with hypermagnesaemia had higher mortality compared to patients with hypomagnesaemia (p<0.001).
In Figure 2 a forest plot with the different hazard ratios (HRs) for mortality are shown, including subgroups. Mortality clearly increased in both hypomagnesaemia (HR 1.86, 95%CI 1.74–1.99, p<0.001) and hypermagnesaemia (HR 1.78, 95%CI 1.48–2.19, p<0.001) compared to normomagnesaemia. Mortality was, irrespectively of magnesium levels, decreased in females and increased in First Nations Australians, in patieets with with eGFR <15 mL/min/1.73 m2, as well as with age.
Table 1: Basic characteristics of 22 293 patients hospitalised and stratified according to the five groups of serum magnesium levels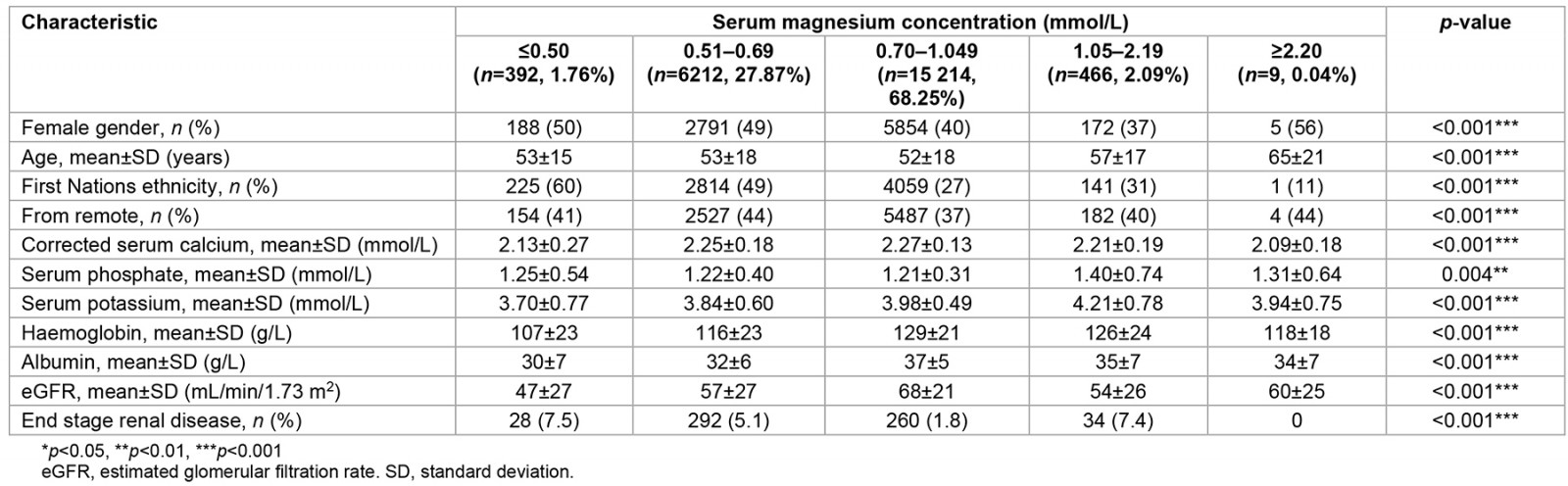
Table 2: Principal diagnosis of hospitalisation in 22 293 patients and stratified according to the five groups of serum magnesium levels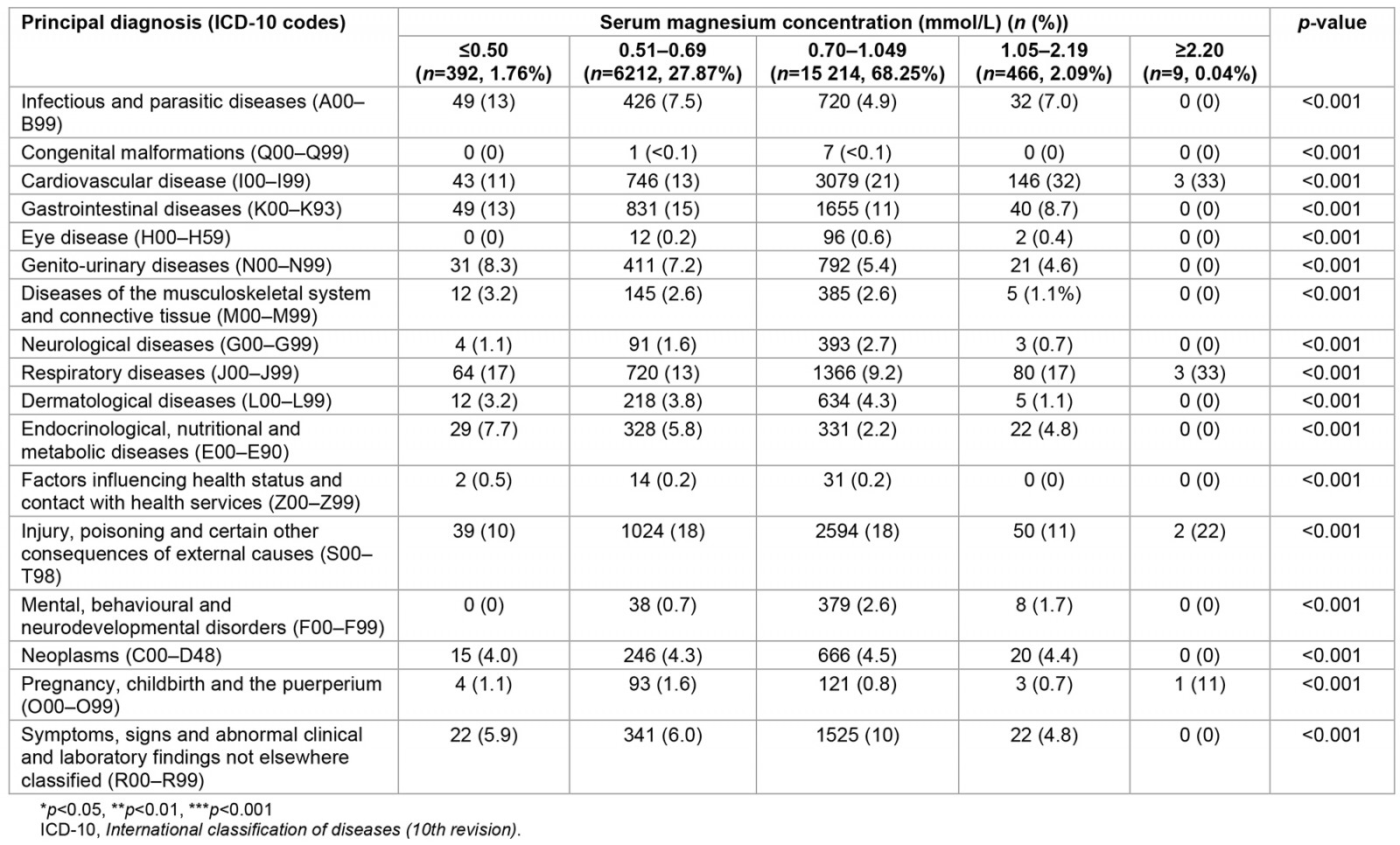
Table 3: Short- and long-term clinical outcomes in 22 293 hospitalised patients and stratified according to the five groups of serum magnesium levels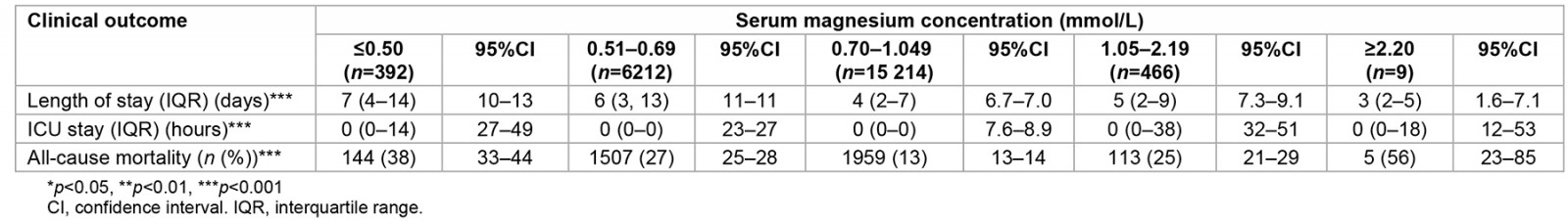
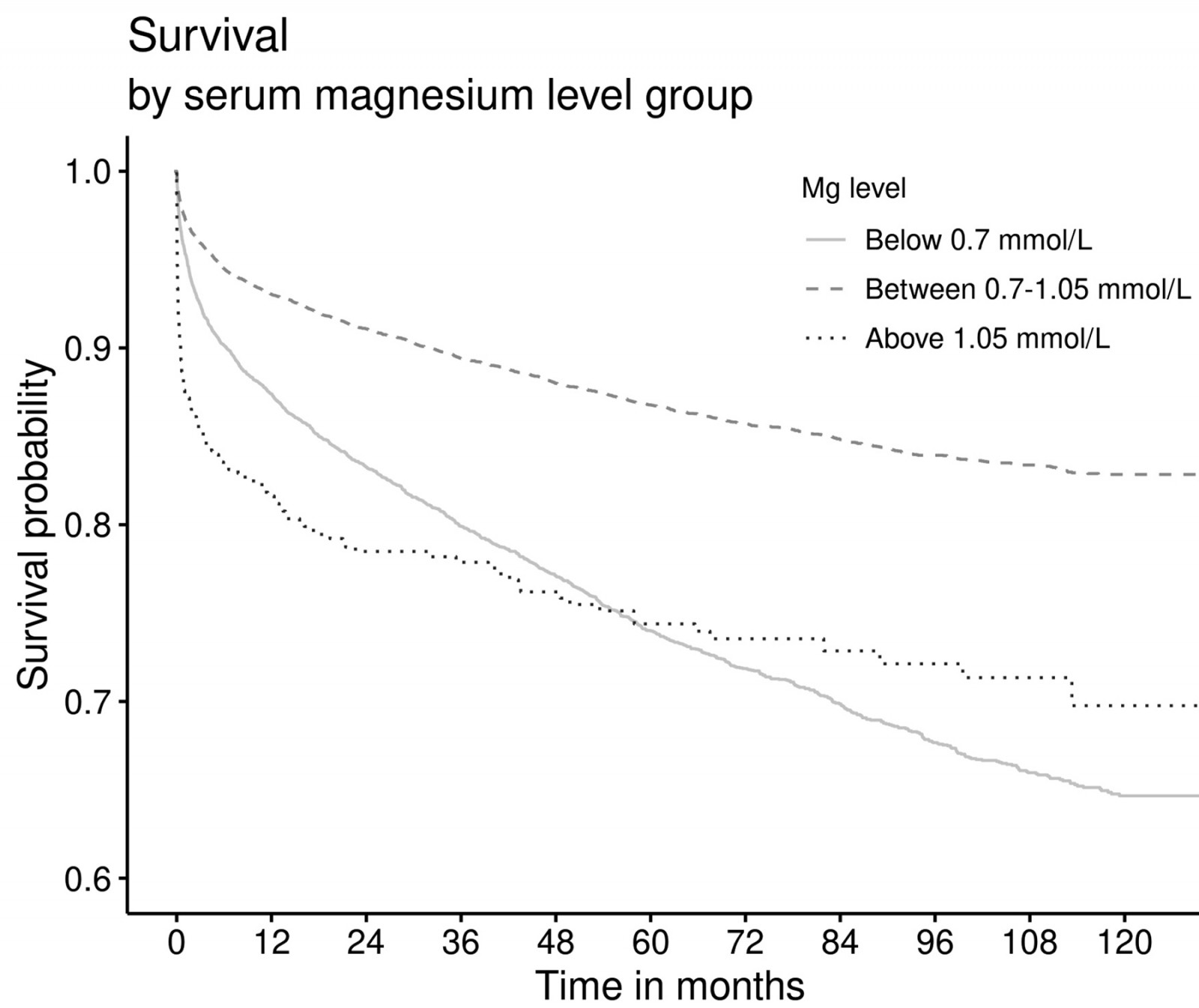 Figure 1: Survival plot stratified according to serum magnesium levels (any hypomagnesaemia <0.7 mmol/L (n=6604), normomagnesaemia 0.70–1.05 mmol/L (n=15 214), any hypermagnesaemia >1.05 mmol/L (n=475)) in all patients hopitalised in the Northern Territory, Australia.
Figure 1: Survival plot stratified according to serum magnesium levels (any hypomagnesaemia <0.7 mmol/L (n=6604), normomagnesaemia 0.70–1.05 mmol/L (n=15 214), any hypermagnesaemia >1.05 mmol/L (n=475)) in all patients hopitalised in the Northern Territory, Australia.
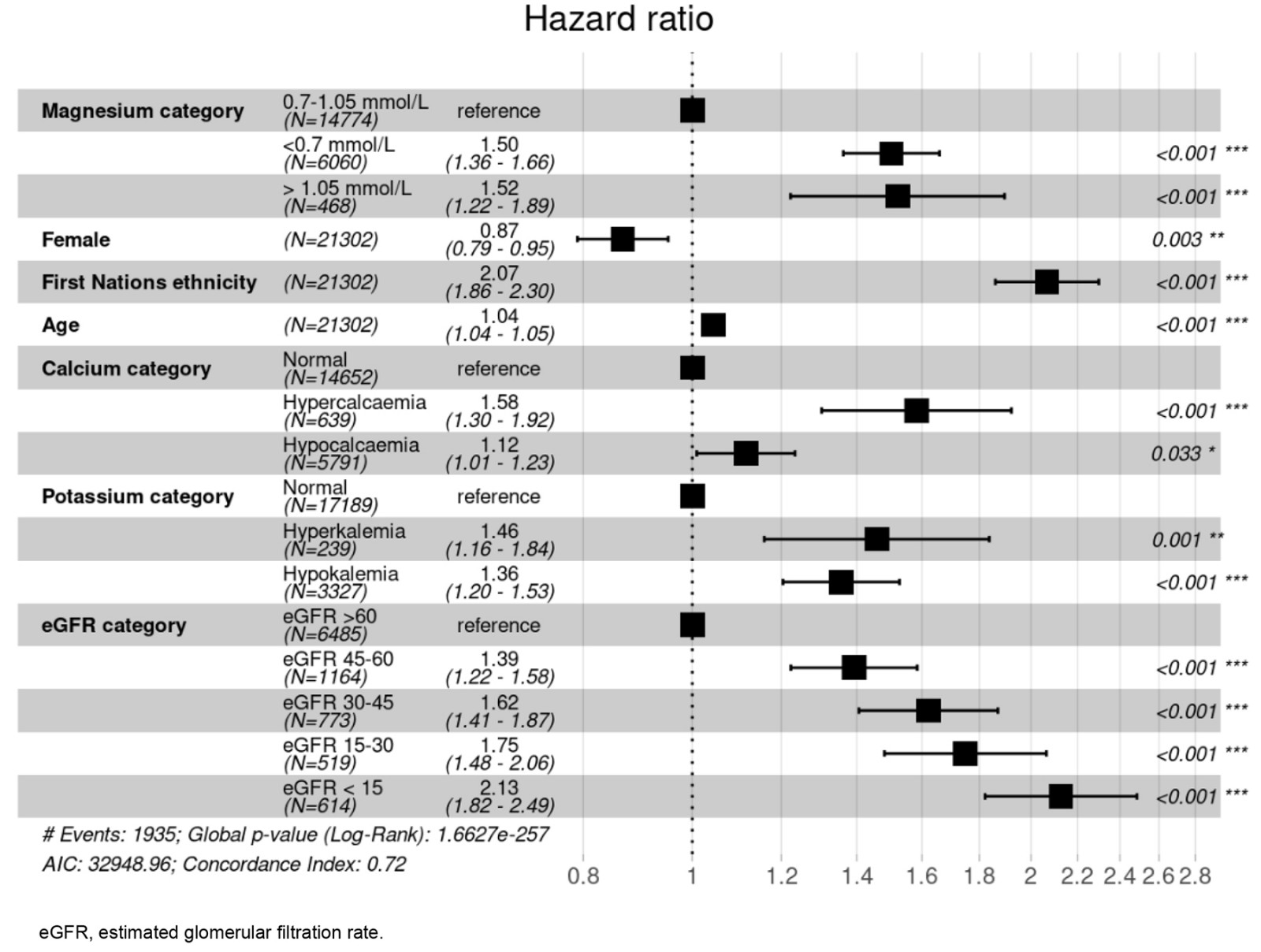 Figure 2: Mortality in 22 293 hospitalised patients with a measured magnesium level. Mortality is shown using a forest plot with the different hazard ratios and 95% confidence intervals as well as p-values.
Figure 2: Mortality in 22 293 hospitalised patients with a measured magnesium level. Mortality is shown using a forest plot with the different hazard ratios and 95% confidence intervals as well as p-values.
Discussion
This was a robust study with a large sample size (n=22 293), including all patients hospitalised in the Northern Territory during 10 years and followed up to death or the end of the study period. We found that dysmagnesaemia was prevalent among hospitalised patients, and hypomagnesaemia was more prevalent than hypermagnesaemia. Hypomagnesaemia, specifically, was very prevalent among the First Nations population. Moreover, dysmagnesaemia was associated with poor health outcomes among hospitalised patients and increased mortality during follow-up compared to patients with normal magnesium levels.
Overall, the prevalence of dysmagnesaemia among hospitalised patients in the Northern Territory (31.75%) was similar to the 28% reported by a study from the US, including adult hospitalised patients17 as well as in another study from China that reported that 27.2% of critically ill patients had dysmagnesaemia18. Moreover, our finding on the prevalence of hypomagnesaemia (29.62%) was similar to what was found elsewhere; a descriptive cross-sectional study conducted in elderly patients with a mean age of 70 years who were admitted to a tertiary hospital found a prevalence of 29% and hypomagnesaemia was more prevalent among the age group of 71–80 years19, and severity of hypomagnesaemia was associated with female sex19,20. In comparison, another study from the US found that 20% of patients with a mean age of 61 years were admitted with hypomagnesaemia21. However, hypomagnesaemia was reported by a few observational studies to be less prevalent than our finding in hospitalised adult patients (7–18%)17,20,22. Hypomagnesaemia was more common in geriatric and critically ill patients (25–65%) and was associated with increased ICU LOS20,23-26 and higher mortality rates24. Although the prevalence of hypermagnesaemia was more common in hospitalised patients with renal failure (10–15%)26, it was less prevalent in other medical patients (1.78–3%)17,20, which is in line with our finding of 2.13%. However, the previously mentioned study from the US found that 7% of patients were admitted with hypermagnesaemia, but the mean age was 65 years, which was older than in our cohort21.
The current study found that hypomagnesaemia was paticularly prevalent among First Nations Australians. Although our study was done in adult patients, a study investigating the food and nutrient intake among First Nations and non-First Nations rural Australian children found that a high proportion of children consumed less magnesium (36%) than the average daily requirements27. Since many of our First Nations patients lived remotely, this may partly explain the high prevalence of hypomagnesaemia in these patients during admission. Overall, people in rural and remote areas of Australia have poorer health outcomes than people living in metropolitan areas. Data demonstrate higher rates of hospitalisations, deaths and injuries, and limited access to primary healthcare services for individuals in non-urban areas than in major cities28.
In addition, a study investigating the magnesium intake of First Nations Australians with type 2 diabetes found that their magnesium intake was lower than the average national intake reported in the National Nutrition Survey29. Type 2 diabetes is very common in First Nations Australians, and it is one of the most common diseases in the world in those living remotely30. About 38% of patients with hypomagnesaemia have been shown to have diabetes mellitus13. Notably, latent long-term magnesium deficiency is common in patients with type 2 diabetes31, and the prevalence of hypomagnesaemia in patients with type 2 diabetes can range from 44.8% to 52%32,33. Alterations in magnesium ion (Mg2+) levels, often in the form of hypomagnesaemia, hinder the translocation of glucose transporter type 4. This interference leads to elevated insulin resistance, influences lipid metabolism, triggers oxidative stress, and disrupts the antioxidant system in endothelial cells. These effects collectively contribute to the onset and advancement of diabetes mellitus and its associated macrovascular and microvascular complications34,35. Unfortunately, we did not have access to comorbities, since we only had access to the principal diagnosis, in the current study so we could not evaluate the prevalence of type 2 diabetes in our hospitalised patients.
Furthermore, high prevelnce of hypomagnesmia among First Nations Asutralians can be attributed to their increased susceptibility to critical illness, severity of sepsis, high poverty rate, and increased alcohol-related disorders and cardiovascular disorders13,36,37. Moreover, we found that dysmagnesaemia was more prevalent in patients from remote areas.
In the present study, we found that infectious, parasitic and gastrointestinal diseases as a principal diagnosis were associated with an increased prevalence of hypomagnesaemia. In comparison, cardiovascular disorders as a principal diagnosis were associated with hypermagnesaemia. Respiratory diseases as a principal diagnosis were very prevalent in patients with both hypo- and hyper-magnesaemia. It has been reported that hypermagnesaemia was associated with increased mortality in patients admitted to respiratory ICU38. An elevation of extracellular magnesium, a crucial intracellular cation involved in enzymatic reactions, occurring concomitantly with the catabolic processes of cells, was associated with acute severe hypoxia38. As mentioned above, hypomagnesaemia is common, especially in people with comorbid conditions such as type 2 diabetes mellitus39. Moreover, hypomagnesaemia has been associated with cardiovascular disease, vascular calcification and endothelial function40. Chronic gastrointestinal disorders affected a third of patients with extreme hypomagnesaemia (<0.3 mmol/L)41. The loss of gastrointestinal fluids, especially in ICU patients, can cause magnesium depletion in conditions like vomiting and nasogastric suction, diarrhoea, enteritis, intestinal and biliary fistulas, inflammatory bowel disease, and pancreatitis22. In addition, hypomagnesaemia was associated with hypokalaemia and hypocalcaemia13,41-43, which aligns with our findings. Certain genetic defects result in renal magnesium wasting and hypocalcaemia, leading to consecutive hypoparathyroidism42,43. Drugs can also be a leading cause of hypomagnesaemia, particularly the use of proton pump inhibitors13,23,41.
It appeared that our patients with moderate to severe hypomagnesaemia also exhibited other critical illness markers, including reduced levels of albumin, eGFR and haemoglobin. Previous studies have demonstrated that hypomagnesaemia is common in critically ill patients and is associated with an increased LOS in ICU, the need for ventilatory support, and an increased mortality rate44,45.
In contrast, hypermagnesaemia is mainly observed in patients with severe renal impairment, especially with creatinine clearances <10 mL/min46. Patients with severe renal impairment can develop life-threatening severe hypermagnesaemia if supplements containing magnesium are administered40. However, we did not find any cases of end stage renal disease in our patients with severe hypermagnesaemia, but the group was very limited with only nine patients included, which may explain the discrepany. Hypermagnesaemia is also typically associated with neuromuscular blockade as well as muscle weakness and can prolong ventilation time24. Hypomagnesaemia, but not hypermagnesaemia, in patients admitted to ICU has been associated with acute kidney impairment development when studied prospectively47. However, there was no association between magnesium levels and the initiation of kidney replacement therapy48. Renal function plays a crucial role in the metabolism of magnesium, and it is practically impossible for hypermagnesaemia to be caused by diet alone in persons with normal renal function26.
Our study showed that dysmagnesaemia was associated with longer LOS and all-cause mortality, which is similar to others17,18,21,24,26,47. A large study from the US using unsupervised machine learning to identify consensus clusters found that patients with the highest comorbidity burden and lowest serum magnesium levels were associated with the highest one-year mortality21. Furthermore, retrospective observational studies showed that both hospital-acquired hypomagnesaemia and hypermagnesaemia measured in non-critically ill adult patients or critically ill patients were associated with higher in-hospital mortality17,18. Moreover, a prospective cohort study from three ICUs in Brazil showed that dysmagnesaemia was associated with higher mortality47. Hypermagnesaemia can lead to concomitant hyperkalaemia, increasing the risk of cardiac arrhythmias and cardiac arrest26. While hypomagnesaemia leads to increased release of endothelin and proinflammatory cytokines, sepsis was found to be an independent factor leading to hypomagnesaemia and therefore increases mortality in critically ill patients24.
Multivariable analysis by a large study showed that hospital-acquired dysmagnesaemia was associated with increased in-hospital mortality with an adjusted odds ratio of 2.1417. Dysmagnesaemia is associated with many serious complications, including cardiac arrhythmia, respiratory failure in patients with asthma or chronic obstructive pulmonary disease, and acute kidney injury, which can explain the association with poor survival. Cardiovascular diseases are mainly linked to hypomagnesaemia leading to the loss of vascular calcification protection and can precipitate acute ischaemia through endothelial dysfunction and hypercoagulability17. In contrast, hypermagnesaemia has been linked with malignant cardiac arrhythmias, leading to cardiac arrest17,49. Moreover, hypermagnesaemia can precipitate neuromuscular dysfunction, respiratory depression and hypotonia, which is commonly found in patients with acute kidney injuries or critically ill patients17. Hence, the higher burden of acute illnesses and comorbidities in the presence of dysmagnesaemia negatively affected survival.
This study has many strengths, including involving all acute hospitals in a huge area with a large sample size and a long follow-up period. It strengthens our understanding of the poor prognostic value of dysmagnesmia among hospitalised patients. In addition, it provides data from the First Nations Australian population as well as from people living remotely. However, limitations include the retrospective design limiting its causal inference; mainly, we were unable to capture data on potential aetiology of the abnormal magnesium levels. Futhermore, the number of patients in the severe hypermagnesaemia group was very limited, making interpretations of this group difficult. Moreover, the study was register-based, and misclassifications may have occurred. In addition, we have not captured some potential confounders that influence the clinical outcomes and survival analysis, including medications known to cause dysmagnesaemia, comorbidities and organ status such as renal and liver profiles. Also, information on the treatment for dysmagnesaemia was not captured, which might influence the LOS both in hospital and in ICU.
Funding
No funding was received for this research.
Conflicts of interest
The authors declare no conflicts of interest.
Conclusion
We demonstrated that dysmagnesaemia was prevalent among hospitalised patients in the Northern Territory. Moderate to severe hypomagnesaemia was more prevalent among the First Nations Australian population. Hypomagnesaemia was associated with a longer LOS, while hypermagnesaemia was associated with a longer ICU stay. Both hypomagnesaemia and hypermagnesaemia were associated with increased mortality.


