Introduction
Maternal morbidity and mortality rates in the US have increased over the past several decades, with higher rates documented in some rural communities with lower access to hospital obstetric services1-3. From 2004 to 2014, 179 rural counties in the US lost all hospital obstetric services, leaving less than half of the remaining rural counties with hospitals that provide obstetric care4. These closures led to greater traveling distances for rural women to access obstetric care, with one study estimating 25% of rural women giving birth at non-local hospitals and 64% of these in urban counties5.
Rural residents possess a 9% greater risk of severe maternal morbidity compared to urban residents6. Severe maternal morbidity is defined by the United States Centers for Disease Control and Prevention (CDC) as ‘unexpected outcomes of labor and delivery that result in significant short- or long-term consequences to a woman’s health’7. Reduced access to obstetric services in rural communities has been implicated in higher rates of morbidity; however, these disparities are likely confounded by differences in social determinants of health and healthcare use surrounding pregnancy6,8-10. Potential confounders include rural–urban differences in education, income, race/ethnicity, occupation, and transportation infrastructure11. Although existing research highlights disparities in maternal outcomes among rural compared to urban residents, it has generally not considered how the location of the hospital influences obstetric outcomes among rural residents. One study linked higher rates of postpartum hemorrhage to rural hospitals with lower delivery volumes but did not control for patients’ geographic residence12. Another study found postpartum hemorrhage was higher in teaching hospitals13, questioning whether patient-level or hospital-level factors are driving this risk increase.
For rural patients, it is unclear whether delivering at an urban facility is protective against maternal morbidity. In this study, we compared obstetric outcomes for rural parturients delivering at urban hospitals versus rural hospitals.
Methods
Data source
We used the data from the Vizient® Clinical Data Base (CDB), used with permission of Vizient, Inc. (all rights reserved) to identify rural patients who delivered from October–December 2015 to October–December 2022. The CDB consists of inpatient and outpatient clinical and administrative records from Vizient member participants, including more than 50 healthcare systems, more than 400 community hospitals, and representing approximately 97% of the academic medical centers across the US. The CDB has been previously validated as a reliable surrogate for institutional medical records in health services research14. To preserve patient confidentiality, ZIP codes and other patient identifying information were not made available to the study team, but a scrambled ZIP code level identifier was generated by Vizient staff to facilitate matching patients within the same ZIP code.
Inclusion and exclusion criteria
Maternal childbirth hospitalizations were determined by the International Classification of Diseases, Tenth Revision (ICD-10)15 diagnosis codes O80*, O82*, and Z37*, or US Medicare Severity Diagnosis Related Groups (MS-DRG) codes 765–768, 774–775, 783–788, 796–798, and 805–807. MS-DRG codes are assigned to patients by insurance companies to categorize their hospitalization based on severity, for the purpose of billing and reimbursement. We included qualifying hospitalizations of patients aged 18–40 years (to limit parturients who by age could be deemed higher risk) who lived in a rural ZIP code, defined as being partially or entirely located in a micropolitan (population 10 000–49 999) or non-core (population <10 000) county16. We limited the dataset to patients arriving from a non-facility location (eg from home) or who were referred to the hospital from a clinic. We then excluded patients who delivered outside of their state of residence, cases with abortive outcomes (ICD-10 codes O00* – O08*), and cases with missing data on study variables. To focus on rural areas where patients have a choice between delivering at urban as compared to rural hospitals, we excluded combinations of delivery year and ZIP code with fewer than 10 rural hospital deliveries or fewer than 10 urban hospital deliveries represented in the dataset.
Variables and covariates
The primary exposure was delivery at an urban hospital, defined as a hospital located in a county with 50 000 or more residents. Distance to the delivery hospital was calculated by Vizient staff based on the ZIP code centroids of patient and hospital address. Hospital teaching status was determined based on hospital membership in the Association of American Medical Colleges Council on Teaching Hospitals and Health Systems. Hospital delivery volume was calculated using all deliveries at each hospital (not limited to individual cases eligible for this study) and was expressed as the average number of deliveries per year, based on all data submitted by each hospital during the study period. Patient-level covariates included year of discharge, demographic data (age, race, ethnicity, primary payor), an indicator for cesarean delivery (ICD-10 procedure codes 10D00Z0, 10D00Z1, 10D00Z2), and whether the patient had high-risk conditions warranting maternal-fetal medicine referral15. These conditions queried included diabetes in pregnancy (ICD-10 codes O24*), hypertensive disorders of pregnancy (O10* – O11* and O13* – O16*), placental complications (O43, O44.00 – O44.33, O44.40 – O44.53), multiple gestation (O30* or Z37.2 – Z37.7), malpresentation (O32*), preterm delivery (O60.1*), and prior cesarean delivery (O34.21 and O66.40).
Study measures
The primary outcome of this study was a composite measure of severe maternal morbidity and mortality (SMM). We hypothesized that, controlling for travel distance, urban hospital delivery reduces severe maternal morbidity among rural women. Patients were categorized as experiencing this outcome if (1) they were not discharged alive from the hospital, (2) they had an ICD-10 procedure code associated with severe maternal morbidity as defined by the CDC17, or (3) they had an ICD-10 diagnosis code associated with maternal morbidity and were transferred to another facility or had a prolonged hospital length of stay suggestive of increased complexity of care (greater than 3 days for vaginal delivery, greater than 4 days for repeat cesarean delivery, and greater than 5 days for primary cesarean delivery). Examples of indicators of SMM according to the CDC include acute myocardial infarction, aneurysm, acute renal failure, acute respiratory distress syndrome, cardiac arrest, disseminated intravascular coagulation, blood transfusion, eclampsia, heart failure, pulmonary edema, sepsis, and hysterectomy17. A sensitivity analysis was performed by limiting the composite outcome definition to exclude cases where blood transfusion was the only indicator of severe morbidity, as it was the most reported maternal morbidity and a potential reason for misclassifying patients as experiencing SMM if they received one or two units of blood with no other complications indicative of SMM. A secondary study aim was to determine if hospital teaching status or delivery volume explained any differences noted in maternal outcomes between urban and rural hospitals.
Data analysis
Data were summarized using medians and interquartile ranges (IQRs) or counts and percentages for continuous and categorical variables, respectively. In the unmatched sample, we compared patient characteristics and outcomes between rural and urban deliveries using rank-sum tests and χ2 tests. In further analysis, we sought to match urban deliveries to rural deliveries by patients with similar characteristics residing in the same rural ZIP code. Therefore, we performed a combination of exact matching on patient ZIP code of residence and nearest-neighbor matching on the propensity score of urban delivery. The propensity model was a logistic regression of urban (versus rural) delivery on all patient characteristics, delivery year, and distance to the delivery hospital. After calculating the propensity score as the linear prediction from this model, we used 1:1 nearest-neighbor matching without replacement, with a caliper set to 0.2 standard deviations (SD) of the propensity score to assure matched cases (urban deliveries) and controls (rural deliveries) were sufficiently similar.
We used propensity score matching to isolate the association of delivery at an urban rather than rural hospital due to our expectation that differences between delivery location are likely confounded by patient characteristics in a complex, multidimensional manner that would be challenging to model accurately with a single-equation regression model. The choice of propensity score matching as an analytic approach is preferred when observational studies are focused on accurately estimating the influence of a single exposure, and when covariate adjustment is performed primarily with the intent of balancing confounding variables between exposed and unexposed cases18.
Urban deliveries without eligible controls, and unused controls (rural deliveries), were excluded from analysis of the matched sample. We checked for covariate balance in the matched sample by calculating standardized differences, where a standardized difference less than 0.1 indicated acceptable balance. In the matched sample, outcomes were compared between urban and rural deliveries using fixed-effects logistic regression, adjusting for any covariates that did not attain sufficient balance between the cases and controls. To address the secondary study aim, we examined how the association between urban delivery and outcomes in this analysis changed after adding control variables for hospital teaching status and delivery volume. All analyses were performed in Stata/SE v16.1 (StataCorp; https://www.stata.com), and p<0.05 was considered statistically significant.
Ethics approval
This study was deemed not to include human subjects research by the Institutional Review Board at East Carolina University.
Results
We identified 803 240 rural patients aged 18–40 years during the study period, of whom we excluded 40 889 patients who were transferred in from another facility, 48 624 patients delivering outside their state of residence, 1258 cases with abortive outcomes, and 43 452 cases missing data on study variables. Of the remaining 669 017 patients, we limited the sample to 214 296 cases from 571 rural ZIP codes that were represented by at least 10 urban deliveries and 10 rural deliveries in the final dataset. This analytic sample included 101 527 (47%) deliveries at a rural hospital, and 112 769 (53%) deliveries at an urban hospital (Fig1). SMM was noted for 2326 cases (1.1%) according to the primary definition that included blood transfusion; and for 557 cases (0.3%) when excluding blood transfusion from the definition of SMM.
Study variables are compared by urban versus rural delivery in Table 1. Urban deliveries involved a higher rate of SMM (primary definition: 1.2% v 1.0% at rural hospitals, p<0.001; secondary definition excluding blood transfusion: 0.4% v 0.1%, p<0.001). Concurrently, urban deliveries tended to involve older parturients; a higher proportion of non-Hispanic Black patients; more patients with commercial insurance; more patients delivering by cesarean section; and a higher proportion of patients with hypertension, diabetes, and history of prior cesarean delivery, as compared to rural deliveries. Urban deliveries were also more likely to involve preterm birth, multiple gestation, malpresentation, and placental complications. Median distance to the hospital was only 5 km for rural parturients delivering at a rural hospital (IQR 0–23), but 43 km for rural parturients delivering at an urban hospital (IQR 31–58). Nearly one-third of urban deliveries, but only 6% of rural deliveries, occurred at a teaching hospital. Among rural deliveries, the median annual delivery volume of the hospital was 630 (IQR 319–962), as compared to a median of 2339 (IQR 1613–3689) for urban hospitals.
In further analysis, we performed propensity score matching to match each urban delivery with the most similar rural delivery by a patient living in the same ZIP code. We excluded 15 283 urban deliveries without a rural delivery control and 4041 rural deliveries not selected as controls during the matching process, leaving 97 486 pairs of urban and rural deliveries in the matched dataset. Covariate balance in the matched data is summarized in Table 2. After propensity score matching, most covariates (including all high-risk conditions) were adequately balanced between urban and rural deliveries. Covariates with residual imbalance included age (older by <1 year in the urban delivery group), distance to hospital (prolonged by 31 km in the urban delivery group), race and ethnicity (higher proportion identifying as neither Hispanic, non-Hispanic Black, or non-Hispanic White in the rural group), and payor (higher proportion with Medicaid coverage and lower proportion with commercial insurance in the rural group). Further analysis of the matched sample controlled for these four covariates to account for the residual imbalance.
Multivariable analyses of the matched sample are summarized in Table 3. Delivery at an urban versus rural hospital was not associated with the risk of any SMM, including blood transfusion (odds ratio (OR) 1.03; 95% confidence interval (CI) 0.91–1.17; p=0.648), but was associated with more than double the odds of SMM other than blood transfusion (OR 2.44; 95%CI 1.81–3.28; p<0.001). After controlling for hospital teaching status and annualized delivery volume (Table 4), we confirmed no difference between urban and rural deliveries on the primary definition of SMM (OR 0.99; 95%CI 0.85–1.15; p=0.873), but a higher risk of SMM other than blood transfusion (OR 1.69; 95%CI 1.21–2.36; p=0.002). In this matched sample, the fully adjusted model (Table 4) indicated being Black, Hispanic ethnicity, having Medicaid compared to a commercial payor, and longer distances to the hospital were associated with the risk of any SMM; older age, having Medicaid as compared to commercial payor, and teaching hospital status were associated with risk of SMM other than blood transfusion.
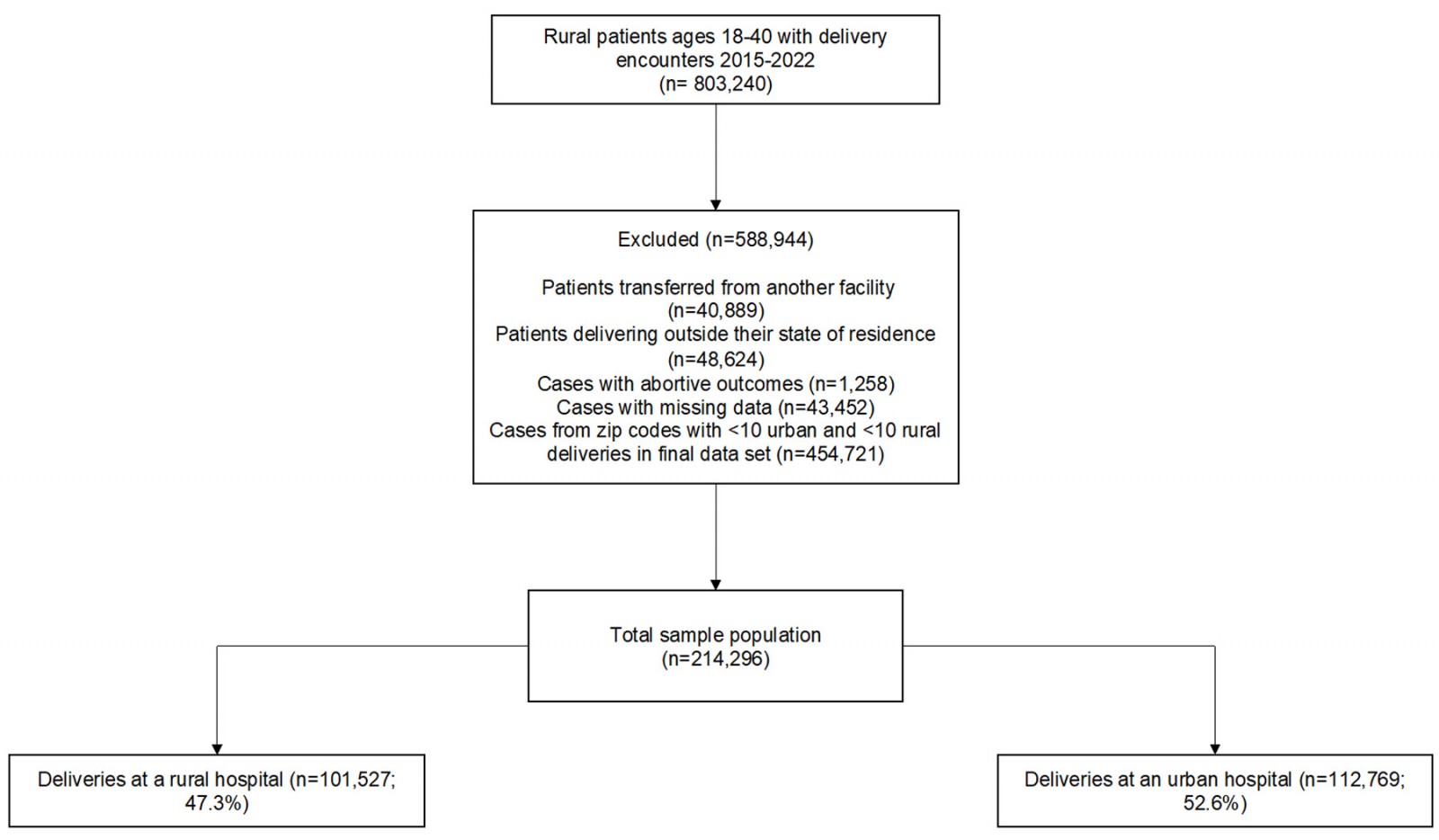 Figure 1: Flow chart of study population.
Figure 1: Flow chart of study population.
Table 1: Comparison of study variables between rural and urban deliveries (N=214 296)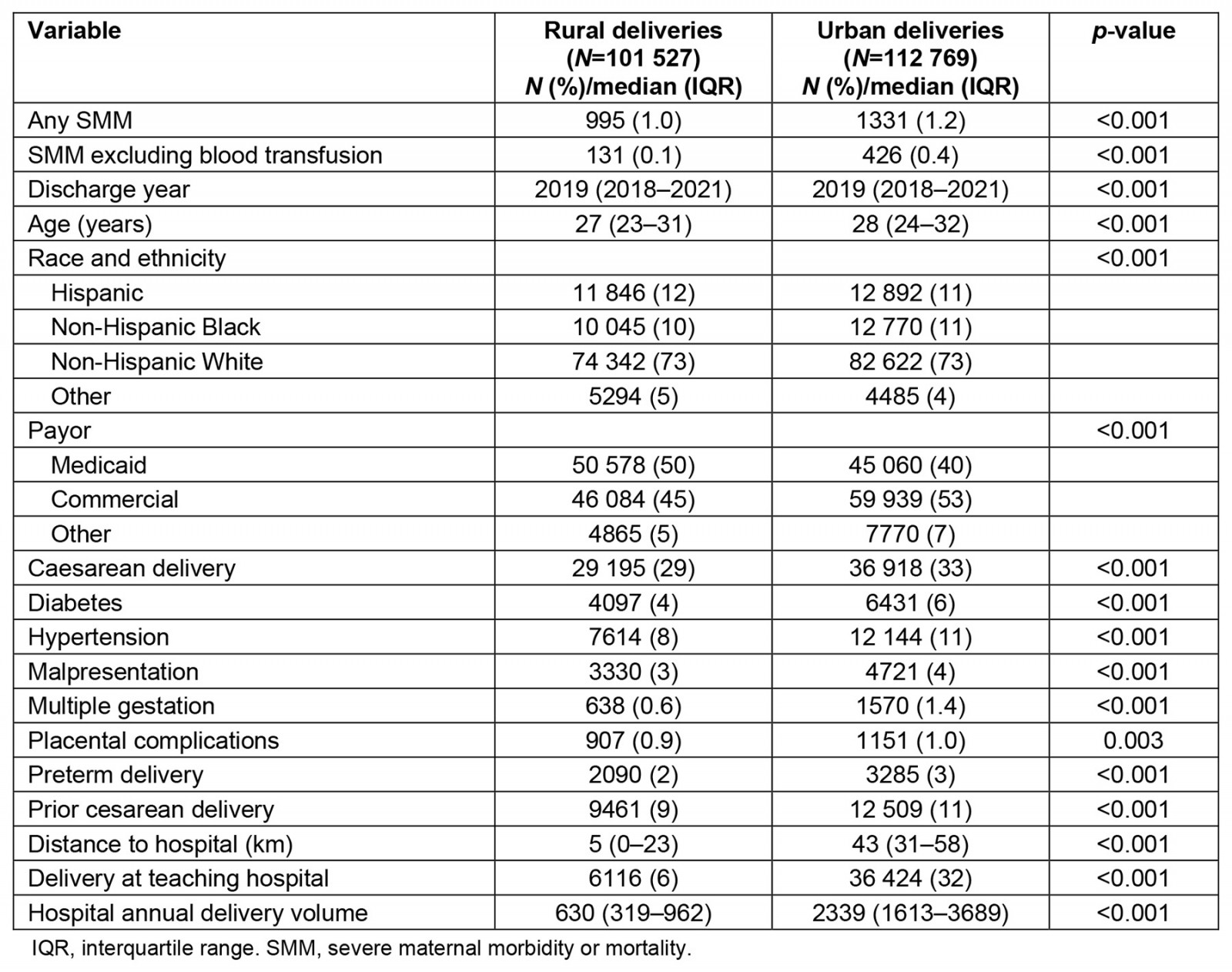
Table 2: Assessment of covariate balance after propensity score matching of rural deliveries to urban deliveries (N=97 486 case-control pairs)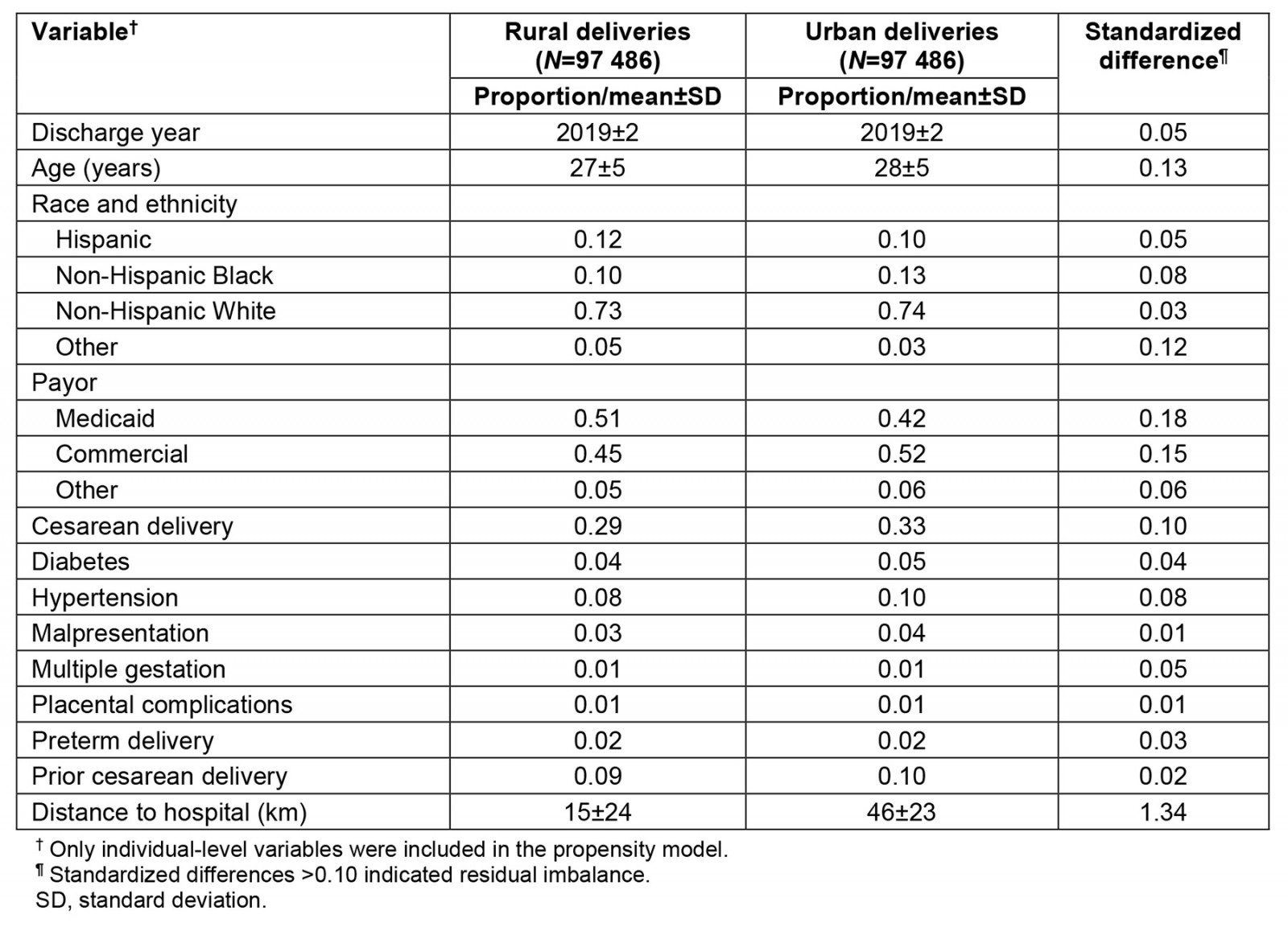
Table 3: Multivariable fixed-effects regression of severe morbidity or mortality on delivery at an urban versus rural hospital, controlling for covariates with residual imbalance after propensity score matching (N=97 486 case-control pairs)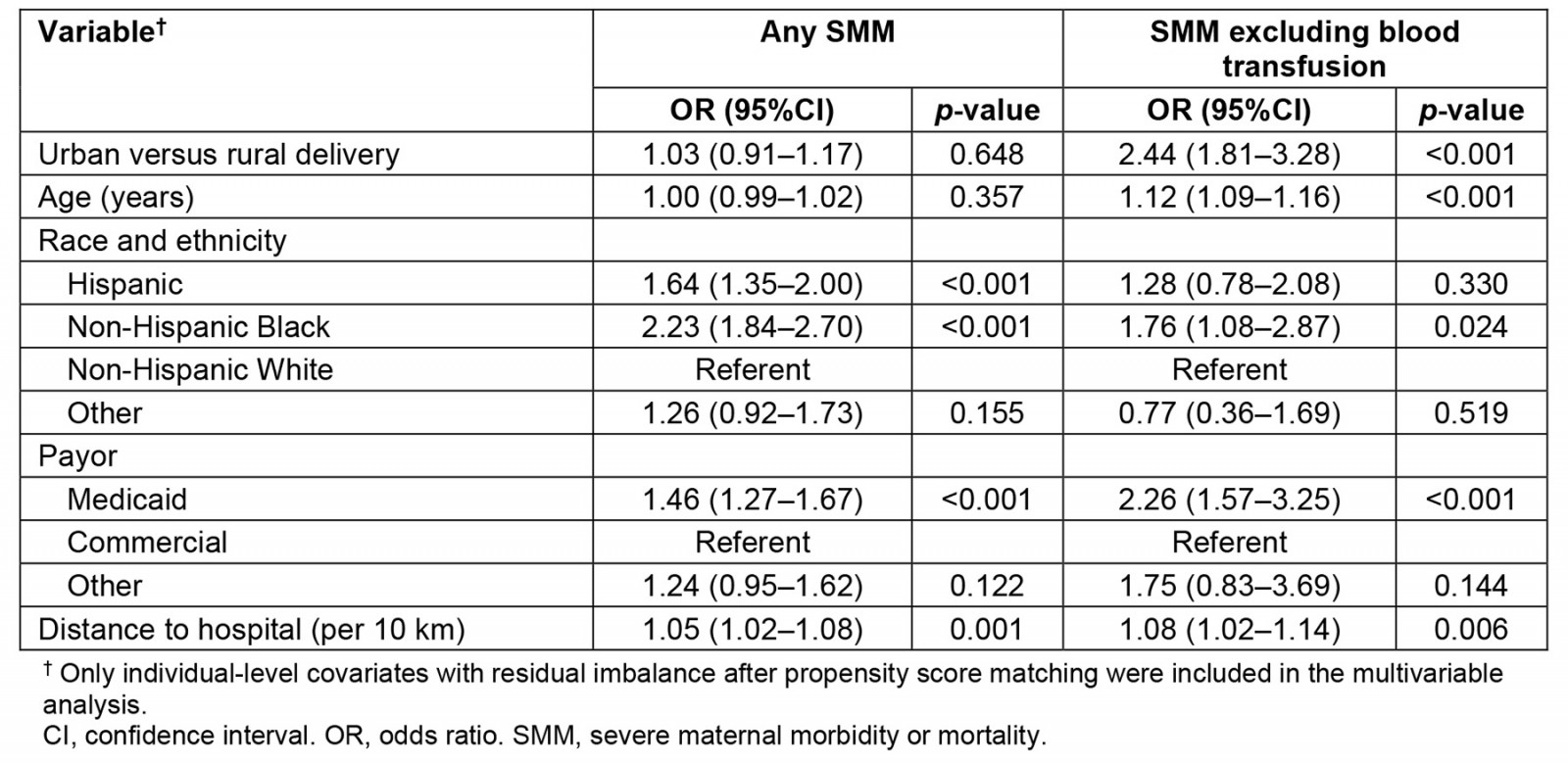
Table 4: Multivariable fixed-effects regression of severe morbidity or mortality on delivery at an urban versus rural hospital, controlling for hospital characteristics and covariates with residual imbalance after propensity score matching (N=97 486 case-control pairs)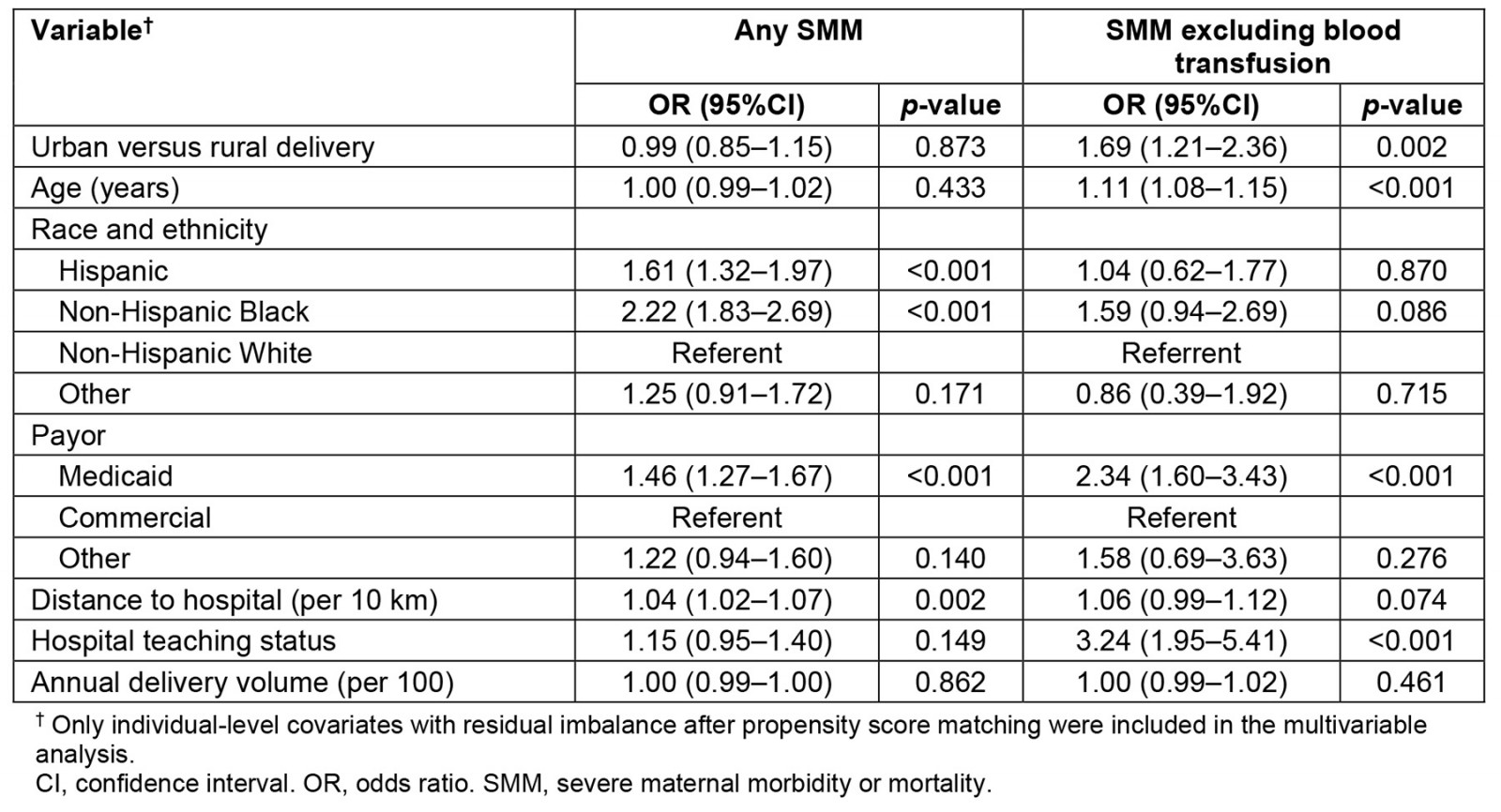
Discussion
Our study explored whether rural parturients experience a higher rate of SMM when delivering at a labor unit in a rural setting compared to an urban one. After exact matching for ZIP code of residence and propensity score matching for individual characteristics associated with the likelihood of delivery at an urban hospital, we found no evidence of reduced SMM rates among rural residents delivering at an urban hospital. Excluding blood transfusions, SMM was more common in rural patients delivering at urban facilities relative to matched rural patients delivering at rural facilities. These findings challenge the assumption that delivery at a rural labor facility possesses inherently greater risks relative to delivery at an urban facility.
In our study there were several characteristics of deliveries at urban facilities that may have contributed to increased SMM. Among urban deliveries there was a higher incidence of conditions associated with an increased risk of SMM (eg older age, cesarean delivery, hypertension, diabetes). The median distance to the delivery hospital was also higher for urban (43 km) versus rural (5 km) facilities, and a longer distance to the delivery hospital has been previously associated with increased maternal morbidity19. Some literature has linked high-volume urban teaching facilities with increased SMM, primarily postpartum hemorrhage13. Our results align with this conclusion, although we note that only one-third of urban deliveries occurred at a teaching hospital in our sample. For our study, urban deliveries were, in most cases, occurring at larger community hospitals with higher median delivery volumes.
When excluding blood transfusion, an increased risk of SMM was no longer observed for Black and Hispanic race compared to White patients. This result is in contrast to other studies documenting higher morbidity among rural and racially minoritized populations in the context of obstetrical care20. Increased efforts are required to fully understand the relationship between blood transfusion and SMM. It is important to note that there is no current standard international definition of SMM, and different definitions exist within the US21. Further research and validation of this measure is necessary to extrapolate data to international populations. Travel concerns are not unique to rural populations, but these populations tend to have increased driving distance to healthcare facilities, which has been linked to increased SMM19 and could partly explain why rural patients delivering at urban facilities appear to have worse outcomes. Rural patients may be more likely to see a non-obstetrical clinician, especially in an emergency context, where some morbidity could relate to unfamiliarity with unique maternal risks (eg pre-eclampsia). Some rural areas, such as eastern North Carolina, have seen a decline in maternal morbidity with implementation of a coordinated program of clinician education and patient pregnancy-status identification22.
After controlling for hospital teaching status and annual delivery volume (Table 4), in this propensity matched sample highest rates for SMM other than blood transfusion were found at teaching hospitals and at urban hospitals compared to rural hospitals. Patients who are high risk may be referred to teaching centers prenatally for care and delivery, which could then increase their risk for SMM. Teaching facilities typically have more providers with different levels of training. Our dataset did not delineate the role of the delivering provider, or any differences in staff credentialing between facilities. SMM was noted to be higher for patients whose payor is Medicaid, a state health insurance plan for individuals with limited income and assets. Low socioeconomic status has been associated with increased risk for numerous pregnancy morbidities23. SMM was increased for non-Hispanic black patients when excluding blood transfusion, aligning with ethnic and racial disparities in maternal morbidity that have been found across the US24. It is important to continue evaluating how discrimination contributes to this disparity in both rural and urban communities. Lastly, it is worth noting that in our regression analysis, odds ratios for covariates (except hospital location) may not be generalizable to a larger population. In a propensity matched sample, covariate balance is achieved when stratifying the sample based on the primary exposure, so we cannot exclude the possibility of incomplete adjustment for confounding variables when interpreting odds ratios of independent variables other than the primary exposure.
Study limitations included our inability to determine the intention behind an individual’s decision to deliver at a certain facility, and whether it was advised due to pre-identified risk factors or if it was the individual’s choice. The Vizient CDB provided a large sample size for our study, and overall SMM rates align with nationally reported data24. However, paid hospital membership to this database is required, and therefore our study sample may not adequately represent hospitals that do not choose (or cannot afford to choose) to participate in this service. The median rural hospital delivery occurred at a hospital with an annual delivery volume of 630 cases, which is substantially higher than many rural hospitals. Inadequate representation of smaller rural facilities may considerably bias the outcomes of our rural sample. Although there may be differences in mortality rates between rural and urban settings, our sample size was not large enough to conclusively analyze mortality as a separate endpoint. We did not explicitly analyze trends in study outcomes, and thus we cannot comment on how the outcomes may have changed over time, with special consideration for any changes in SMM during the COVID-19 pandemic. The structure of this database also limited our ability to reliably control for parity in SMM outcomes, and our analysis of the matched sample did not include all potential risk factors for SMM.
Conclusion
Our findings have significant implications in how we choose to provide maternity care in rural regions, but the landscape of rural health care is nuanced and unlikely to be captured in a single dataset. Our study and previous literature demonstrate that rural patients have better maternal outcomes when they receive care locally. Making that possible will require significant efforts to navigate the complexities of rural medicine and to create innovative and sustainable solutions to maintain access to care for individuals living in these communities.
Funding
No funding was received for this research.
Conflicts of interest
The authors declare no conflicts of interest.
References
You might also be interested in:
2020 - Can neonatal pneumothorax be successfully managed in regional Australia?
2007 - Evaluating Australian Indigenous community health promotion initiatives: a selective review

