Immunisation programs are one of the success stories in international health1,2. While there has been significant progress in immunisation coverage in all countries through WHO's Expanded Program on Immunization (EPI), this has not been consistent, with around 19.3 million infants failing to be immunised in 20102,3. Vaccination rates (VRs) have been particularly patchy and low among poor, rural and marginalised groups1,2. Reasons for this limited success can be attributed to features within both the vaccination programs and the target populations1,2.
Like many other developing countries, Tanzania has witnessed some improvement of national immunisation rates over the past decade. While immunisation rates for the individual vaccines bacillus Calmette-Guérin (BCG), three doses of diphtheria-pertussis-tetanus (DPT3), three doses of poliomyelitis (Polio3), and measles have varied nationally from 72% to 90% from 1998 to 2007 and have increased in general4,5, the three Tanzanian surveys from 1996, 1999 and 2004-2005 indicate a rather low FVR (all vaccines received by 1 year of age), with virtually no increase over the years (1996: 71%; 1999: 68%; 2004-2005: 71%)6-8 and being well below the WHO targets of >80% coverage in an administrative district and >90% national coverage1.
The published reports on the vaccination status of infants in Tanzania do not provide detailed data on the infants in difficult-to-reach populations in rural, remote communities. In these areas (and among poorer and less educated families, which are often in the same groups), improvement has been slower9. For the Arusha region in northern Tanzania, which includes some parts of the Mbulu area, the investigators' main service area, 80% FVR (based on 53 out of ~56 000 infants) was reported in 2004-2005, while for the Manyara region, which includes the Mbulu district as part of the Mbulu area, the corresponding figure was 74% (based on 57 out of ~51 000 infants)8,10.
Nomadic pastoralists, who represent a large proportion of the population in the Mbulu area, present a special problem to immunisation services as they are mobile for almost all of the year and hard to reach11-14. They place a high value on the wellbeing of their livestock whose health and survival determine the wealth, health and survival of the family and the tribal members11-14. Thus the necessity to seek preventive and curative health care for their children and to vaccinate them is not always seen as a priority. Data from pastoralist groups in Chad showed that the vaccination coverage can be almost zero in this population11,12.
The objectives of this retrospective study were to determine the VRs for the individual vaccines BCG, poliomyelitis, DPT and measles and for all vaccines together (full vaccination rate (FVR)) of all infants (<1 year) who were registered at eight mobile reproductive-and-child-health (RCH) clinics run by the local hospital in the remote, rural Mbulu area in northern Tanzania during the years 1998, 1999, 2006 and 2007; and to analyse differences in individual VRs and FVRs according to clinic and year. The study also examined possible underlying factors, including predominant tribal affiliation at the clinic site, rates of skilled birth attendance, service provision and vaccine availability. The data was expected to help inform immunisation programs so that they could address any identified gaps in immunisation coverage in these vulnerable populations, and support local and district health authorities to restructure and improve immunisation services especially for disadvantaged clusters within the population.
Setting
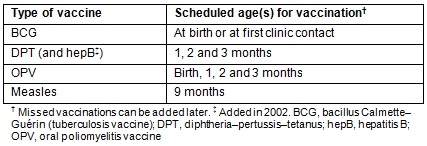
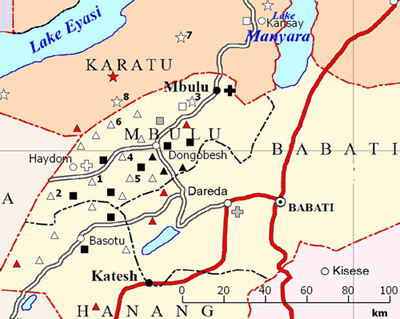
The study was based at the facilities and RCH clinics of Haydom Lutheran Hospital (HLH), a rural church hospital in northern Tanzania located at the southern edge of Mbulu district in the Manyara region (Fig1)15-19. The 400-bed hospital serves more than half a million people in the wider catchment area, providing surgical, medical, gynaecological/obstetric and paediatric services15-29. Each year, more than 12 000 inpatients and 70 000 outpatients were treated, and 2200-3500 children were born at the hospital17,20-29, while more than 11 000 were born at home in the catchment area10,15-17,20-29. During the first 2 years of this study (1998-1999), 20 mobile RCH clinics were run by the hospital and located up to 100 km from HLH. In addition, other RCH clinics were operated by government and voluntary agencies in the same area (Fig1). In 2006 and 2007, the number of HLH-affiliated mobile RCH clinics had increased to 2715-17,28,29. These clinics, using a four-wheel-drive vehicle or a light aircraft for more remote locations and conducted by HLH staff, were held at fixed locations at regular dates once per month which were made known to the local population in advance. In addition, a permanent RCH clinic was open daily at HLH. Every year, these clinics conducted over 25 000 antenatal care examinations in pregnant women and over 65 000 examinations (including immunisations) in children younger than 5 years15-17,20-29. During the clinic sessions, regular educational sessions on various health topics were provided. The Tanzanian child immunisation schedule for the years 1998, 1999, 2006 and 2007 is shown in Table 17,30. In the year 2002, hepatitis B vaccination was added to the national EPI program, which is organised as a governmental, centralised procurement and top-down distribution system of vaccines, and co-administered with DPT in a combination vaccine, but the overall schedule remained the same30.
Table 1: Tanzanian child immunisation schedule for the years 1998, 1999, 2006 and 2007
Population characteristics
The Mbulu area, located in the southern Karatu district and the Mbulu district (Fig1), is a rural, difficult-to-reach area with poor road infrastructure8,15-17,19. Its population is unique in that it comprises several different language groups with distinctly differing ways of life13,15,16,31-34. The major tribes are the Iraqw with a population of around 500 000, who are mainly subsistence farmers with some domestic cattle15,16,31,33,35,36; and the Datoga, who number around 100 000-150 000 people and are nomadic pastoralists, moving around with their livestock over long distances13,14,31,33,35. The third and smallest group, the Hadza hunter-gatherer tribe, numbers only 1000-1500 people33,34. The Iraqw mainly populate the highland plateau of the Mbulu and Karatu districts15,16,31,33,36. The Datoga and Hadzabe reside in the Yaeda Valley between Lake Eyasi and the eastern escarpment of the Rift Valley, with the Datoga additionally occupying areas south of the HLH catchment area in the Basotu division of Hanang district13-16,32,33. The remaining tribes belong to the Bantu who are typically subsistence farmers or small-scale traders15,16,33. The Datoga and the Hadzabe are difficult to reach with any kind of social services, including healthcare provision by the mobile RCH clinics13,14,32,34. In contrast, the Iraqw and Bantu communities are more easily accessible at these clinics32,33,35,36.

Data collection
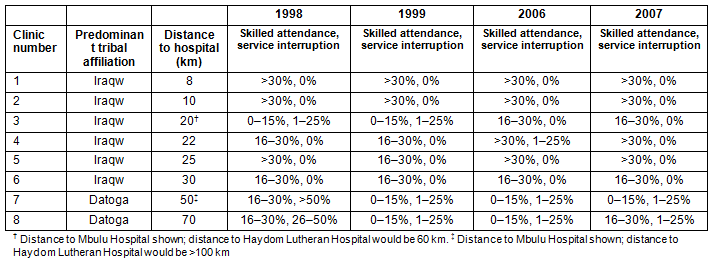
Eight RCH clinics (clinics 1-6 located in the Iraqw mainland; clinics 7 and 8 located in the Datoga mainland) (Table 2; Fig1) were chosen for the study to represent the characteristics of the RCH clinics (location in the Iraqw or Datoga mainland, distance to hospital, remoteness, access by car vs plane). The number of clinics increased from 20 to 27 during the study period, but none of the new clinics was added to this analysis to ensure comparability between the two periods. A retrospective, cross-sectional study design was used to compare vaccination data of the years 1998, 1999, 2006 and 2007 that had been collected by the RCH clinic staff as part of the reporting requirements for the national EPI program.
All registered infants from the eight sites were included in the analysis. Infants were only registered at one clinic and their mothers received vaccination cards, where all vaccinations were recorded in addition to the clinic records. The main dependent variable was the number of vaccinations per registered infant during the first year of life. Independent variables were collected additionally and included:
- major tribal affiliation at each RCH clinic. Data on tribal affiliation were not provided on an individual basis in the records according to government regulations, so each RCH clinic was assigned a major tribal affiliation based on the predominant tribe (>80%) that used the clinic services (Table 2; Fig1)
- distance from RCH clinic to main healthcare provider (HLH or other hospital) (Table 2). This parameter was not used for statistical analysis, but should demonstrate the remoteness of some of the clinics (Fig1)
- skilled attendance status at birth of each registered infant (at a hospital, health centre, dispensary or at home). All births in health institutions (dispensary, health centre, hospital) were deliveries with skilled attendance (Table 2). At home, births were attended by traditional birth attendants, relatives or no attendants at all
- service provision at the RCH clinics (Table 2), defined as the provision of immunisation services regularly once a month. Reasons for not providing the service were inaccessibility of the clinic sites by car or plane due to bad road or airstrip conditions
- vaccine availability at the RCH clinics, which would affect all sites on schedule during the time of vaccine shortage (see later for further explanation in relation to Table 6).
Table 2: Predominant tribal affiliation, distance to hospital, skilled attendance
at delivery and degree of service interruption at reproductive-and-child-health clinics
Data analysis
All data were entered manually in data collection sheets, checked for inconsistencies and then entered into the Stastical Package for the Social Sciences v15.0 (SPSS, Inc.; http://www.ibm.com/software/analytics/spss‎) for analysis. The VR at the different RCH clinics was calculated for each single vaccine type and for full vaccination before the first birthday as follows: (number of vaccinated registered infants at RCH clinics ÷ number of all registered infants at RCH clinic) × 100. Differences in VRs (for the single vaccines and for full vaccination) between the different RCH clinics and between the 4 years of study were analysed with the χ2 test and univariate logistic regression for calculation of odds ratios and confidence intervals. To correlate the outcome variable VR at the different clinics and over the time periods with possible underlying factors, both univariate and multivariate forward logistic regression analyses were performed, adjusting for predominant tribal affiliation at the clinic site and skilled attendance at birth. The independent variables - predominant tribal affiliation, deliveries with skilled attendance (both related to the target group) and service provision (provider-related) - were tested with the FVR as the outcome variable. For the variable vaccine availability (provider-related), the respective single VR was used as the outcome variable. Odds ratios (OR) with 95% confidence intervals (CI) were calculated by these analyses. The level of significance (two-sided) was defined as p<0.05.
Ethics approval
Ethics approval for the study was granted by the National Institute of Medical Research (approval number NIMR/HQ/R.8a/Vol. IX/824) and the Commission for Science and Technology (approval number 2010-60-NA-2009-02) in Tanzania and the Human Research Ethics Committee at Curtin University (approval number CIH 8-2008) in Australia. Permission to use the RCH records was obtained from the HLH management.
In all, 3868 infants (1941 male, 1927 female; p=0.822) were included in the analysis (Table 3). The annual FVRs varied between 57% and 72% and showed a significant difference between the highest rate in 1998 and the remaining 3 years (OR = 1.83 (95% CI 1.55-2.16)) (Tables 3,4). Looking at the combined VR for BCG, DPT3 and Polio3 (65-84%), but excluding measles vaccination, there was a significant difference between the rates in 1998, 2006 and 2007 and the lowest rate in 1999 (OR = 2.10 (95% CI 1.79-2.47)) (Tables 3,4). These patterns persisted when clinics 7 and 8, with the lowest FVRs, were excluded from the analysis (Tables 3,4).
For all years, VR for BCG was 93%, for DPT3 82%, for Polio3 80%, and for measles 66% (Table 3). For most of the single vaccines, there appears to have been a reduction in the VR over the years (Tables 3,4). The Polio3 VR increased over the period, with it being lowest in 1999, and achieving better results in 1998, 2006 and 2007 (OR = 2.62 (95% CI 2.21-3.09)). Measles VR was consistently the lowest among all the vaccine types, except for 1999 when Polio3 VR was even lower. FVR was determined by measles VR, except in 1999 when the Polio3 VR affected the FVR even more (Table 3).
Full vaccination coverage as defined by WHO (at least 80% in each administrative unit) was only achieved at clinic 6 during three of the 4 years (Table 3). At no clinic was a consistent increase observed in individual VR or FVR over the study period. The RCH clinics 7 and 8 showed the lowest results for FVRs and individual VRs of the single vaccines (OR = 4.55 (95% CI 3.76-5.48)) (Tables 3,4).
The FVR was lower at the clinics predominantly consulted by the Datoga tribe compared to the Iraqw tribe (OR between 3.33 and 4.97) (Table 5). Combining all years, RCH clinics with low rates of deliveries with skilled attendance (Table 5) tended to have lower FVRs compared to those with intermediate and high rates of skilled deliveries (OR= 3.56 (95% CI 2.91-4.36)). RCH clinics with intermediate rates of skilled deliveries had higher FVR results than RCH clinics with the highest rates (OR = 0.83 (95% CI 0.72-0.96)) (Table 5). The results for single years were more variable (Table 5).
On the provider side, interruption of monthly service provision at the respective RCH clinics (Tables 2,5) was a significant risk factor for low FVRs (Table 5). Even an interruption of up to three service dates per 1 year (<25%) affected the FVR adversely. Interruption of monthly service provision occurred mainly at the RCH clinics that had a predominant Datoga tribal affiliation (except for clinic 3 in 1998, 1999 and 2007, and for clinic 4 in 2006) (Tables 2,5).
Interruptions in the regular supply of vaccines had a detrimental effect on VRs (Table 6). For each single vaccine the degree of lack of vaccine supply corresponded closely with the VRs throughout the years. Bacillus Calmette-Guérin coverage dropped in 2006 and 2007 when the supply of BCG vaccines was interrupted for around 30 days in 2006 and for around 15 days in 2007. The oral polio vaccine was not available for more than half a year during the period 1999-2000 with a resultant marked drop in the VR, particularly for 1999.
For the DPT vaccine, the picture was more variable, but overall periods of interrupted vaccine supply closely corresponded to changes in DPT3 VRs, being the lowest in 2007. For measles vaccination, there was a correlation between diminished vaccine availability (lack of supply less than 15 days) and low VRs in 2006 and 2007. FVR also reflected the measles VR (overall measles VR being the lowest of all four vaccine types). This is unlike the other three vaccines, where a temporary drop in the VR due to interrupted vaccine supply did not affect the overall FVR (except for Polio3 in 1999).
The adjusted OR (AOR) for predominant tribal affiliation at the clinic sites remained significant, demonstrating its strong effect on FVR, while the AOR for skilled attendance at delivery became insignificant in 2007 and for all years together (Table 5).
When looking at the influence of predominant tribal affiliation and delivery with skilled attendance on interruption of service provision, a consistent finding was that adjusting for tribal affiliation lowered the AOR in general, sometimes leading to an insignificant ratio (1998 and 2007). The influence of delivery with skilled attendance and of both parameters together on the AOR was not consistent (Table 5).
When analysing the AOR for the single vaccines BCG, DPT3, Polio3 and measles, some small changes could be observed for adjusting with predominant tribal affiliation and delivery with skilled attendance, but the main effect of lack of vaccine supply on the single VRs was not altered throughout the analyses (Table 6). This demonstrated a strong effect of interrupted vaccine supply on achievable VRs.
Table 7 summarises the risk factors identified as predictors for poor vaccination coverage rates at the different RCH clinics. Clinics 7 and 8 were particularly vulnerable to risk factors, followed by clinic 3. These were the clinics with the lowest VRs and FVRs. Strong predictors for lower VR and FVR were predominant affiliation with the Datoga tribe, low rate of deliveries with skilled attendance and frequent service interruptions. Lack of individual vaccines determined differences in the vaccination rates of these vaccines between the years, and was a potent factor for the respective VRs.
Table 3: Vaccination rates at reproductive-and-child-health clinics 1998, 1999, 2006 and 2007
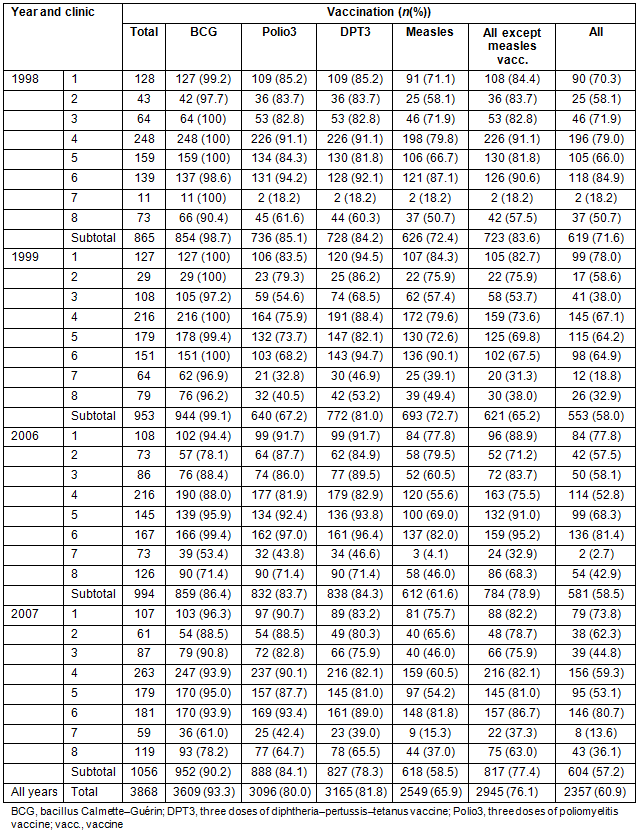 Table 4: Analysis of vaccination rates over the years and across the different study sites
Table 4: Analysis of vaccination rates over the years and across the different study sites
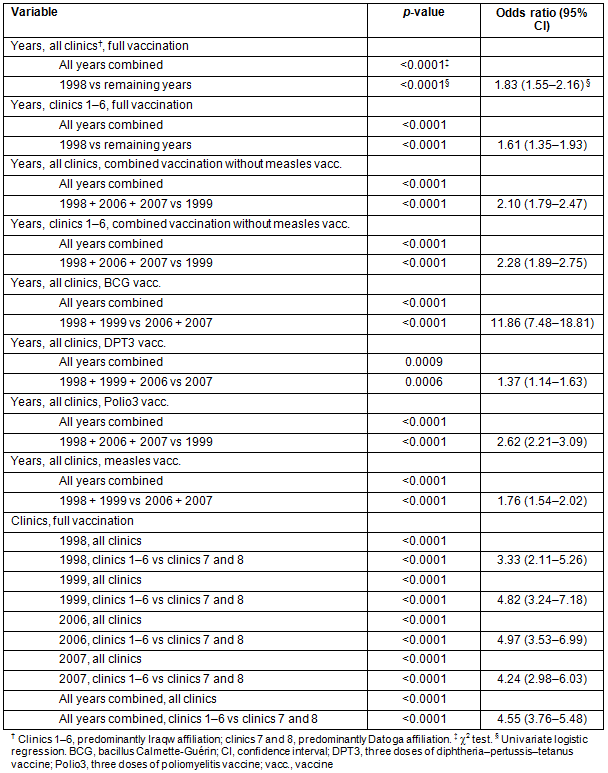 Table 5: Univariate and multivariate logistic regression analyses for the dependent variable 'full vaccination status'
Table 5: Univariate and multivariate logistic regression analyses for the dependent variable 'full vaccination status'
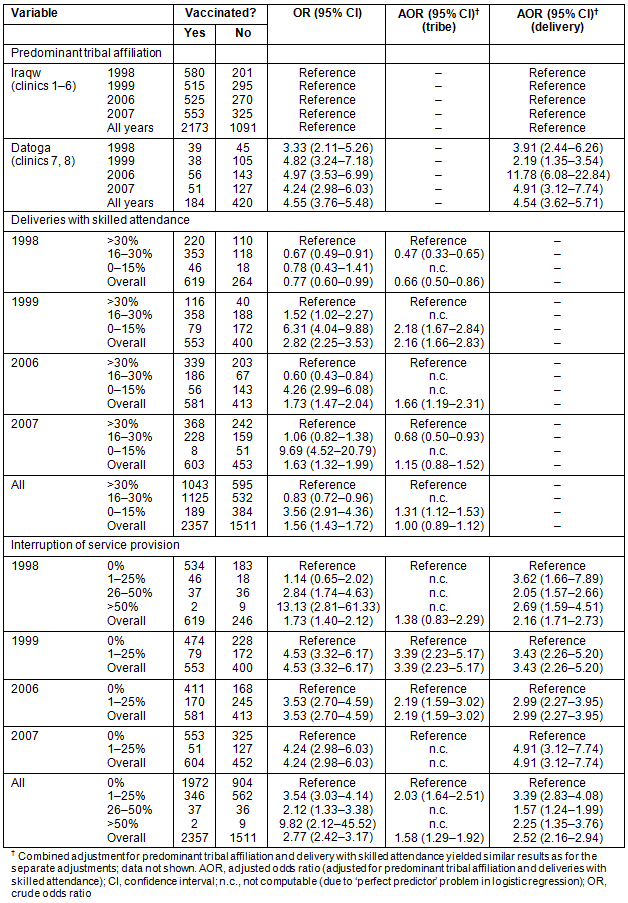 Table 6: Univariate and multivariate logistic regression analyses for the dependent variable 'single vaccination status'
Table 6: Univariate and multivariate logistic regression analyses for the dependent variable 'single vaccination status'
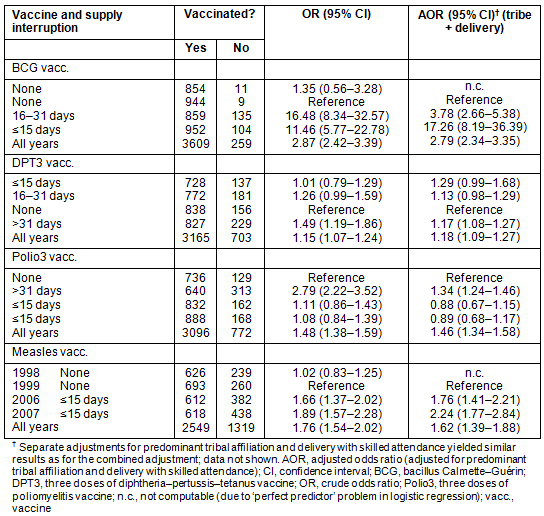 Table 7: Distribution of identified risk factors at the eight reproductive-and-child-health clinics
Table 7: Distribution of identified risk factors at the eight reproductive-and-child-health clinics
Discussion
This study provides a detailed analysis of vaccination coverage rates and their associations during the first year of life among a difficult-to-reach population in a remote area in rural Tanzania. While some of the sites in the study had similar annual FVRs to the rural and national FVRs, at other sites they were much lower than national figures6-8 and rarely reached or exceeded the 80% WHO district target1. Although the rates for single vaccines were considerably better than the FVRs, they were still too low, particularly for measles and for Polio3 in 1999. In a study from rural southern Tanzania during 2004, VRs for BCG were 89% (our pooled data: 93%), for DPT3 81% (82%), for Polio3 91% (80%), and for measles 69% (66%)37. Similar studies have been published from South Africa38,39, Uganda40 and Bangladesh41, which found that late vaccinations such as the third dose of DPT or measles were consistently affected and that timely administration of the vaccines was frequently delayed39,40.
Another area of concern was the finding that VRs would not increase over the study period, but in fact decreased at several sites. This parallels to some extent the results of the Tanzanian surveys between 1996 and 2005 with stagnating FVR between 68% and 71%6-8. These data indicate first that there is considerable room for improvement of immunisation coverage in difficult-to-reach populations, and second that national immunisation figures can give a misleading impression of the vaccination status of sub-groups like nomadic or remote rural populations.
Whether the national Tanzanian vaccination data represent over-estimates of vaccine coverage as suggested recently by Lim and colleagues cannot be confirmed by this study, but their results might indicate that the findings of this study are closer to real vaccination coverage than official data42. However, national immunisation days can considerably help in improving vaccination coverage. In 1999 and 2000, the Tanzanian government conducted national immunisation days for improving poliomyelitis vaccine uptake43. Thus it may be possible that the actual figures for Polio3 VRs in 1999 were considerably better than documented in the RCH records as these national vaccination days were not recorded in the same system.
The retrospective approach of this study using secondary data meant that the possibility of some infants receiving missing vaccinations at other RCH clinics while the families were moving from one place to another cannot be excluded. But if these infants attended at a later date the original RCH clinic where they had been initially registered, then vaccinations received at the other clinic and recorded on their vaccination card would have been transferred into the records of the original RCH clinic. As the vast majority of mothers and infants have to walk to the mobile clinics meaning 1-2 hours travelling time for one direction on that day, it is not very likely that they would have changed the clinic site frequently.
A potential unexpected explanation for the low documented measles VR was revealed by informal inquiry. Health staff observed several times that mothers would not return to the registration desk after having their infants vaccinated with the measles vaccine, the last one in the schedule, but would leave the RCH clinic immediately. These vaccinations were not documented in the infants' RCH records and were neither available nor quantifiable in this retrospective analysis. Thus, the registration system at the RCH clinics needed to be modified so that vaccines that were administered would reliably be recorded for each infant. This change has consequently been implemented.
Measles VRs could be 10-20% higher then, giving a potential for considerably higher FVRs. This would explain why so few cases of infants and children with vaccine-preventable diseases were seen at HLH, the main health institution in the area, despite quite a low documented coverage, although up to 50 measles cases were diagnosed in some of the years20-29. However, this observation does not explain the differing VRs of the other vaccines as they were given on previous RCH clinic visits, were at least documented on the child's own vaccination card carried by the mother, and could be recorded in the RCH records on the following visits to the clinic.
Due to its retrospective nature, this study could not document infants registered in the RCH files at their first contact who were lost due to late neonatal mortality or death during infancy. When considering data from UNICEF4,5 and the Tanzanian surveys6-8, infant mortality in Tanzania was 70-90 deaths per 1000 live births, and 10-15 more deaths per 1000 live births in rural areas. Thus the number of surviving infants at the RCH clinics could well have been smaller by 8-10%. This could increase VRs and FVRs considerably for the surviving infant cohort.
Reproductive-and-child-health clinics in the nomadic Datoga mainland had the lowest VRs throughout the years. Therefore the effect of underlying factors like tribal affiliation and utilisation of health services (measured as levels of skilled birth attendance as a proxy) on VRs and FVRs was analysed. Univariate analysis showed that differences between the RCH clinics were well explained by predominant tribal affiliation at the sites. Infants from predominant Datoga-affiliated sites had markedly lower immunisation coverage, both for single vaccines and FVRs. Incidentally, these were the sites located farthest from the hospitals in the area (Table 2; Fig1). Whether geographical distance really explains these differences is questionable, as mobile clinics were held close to the population. A better explanation would be the nomadic lifestyle of the Datoga, rendering them less accessible. The wellbeing of their livestock is of major importance for the tribe's survival and wealth, thus they may forego immunisation visits to an RCH clinic in favour of livestock survival11,12.
Anthropological research suggests that the Datoga are not well reached by the official health system, which they do not perceive with confidence13-16. This view is supported by the finding that the rate of deliveries with skilled attendance, which was used as a proxy for contact with the formal health system, was quite low among the Datoga sites compared to the other sites. Preliminary results from another anthropological study indicate that the Datoga do not place a high health priority on vaccination, and perceive interaction with health staff as difficult44. Similar findings were reported from nomadic populations in Chad11.
As the Tanzanian EPI program is a national public health priority with a centralised procurement and distribution system of vaccines, lower-level health facilities like the RCH clinics run by HLH depend on good management and uninterrupted supply to administer this program continuously. Great effort was taken by the RCH staff to calculate correctly the amount of vaccines and other supplies needed and to order these well in advance from the central district office. In addition, it was deemed crucial to adhere strictly to the monthly RCH schedule, which people relied on. Nevertheless, due to difficulties in the timely and adequate supply of vaccines and due to unforeseeable problems to reach the RCH clinic sites on the given day (like impassable roads in the rainy season or no plane available), at times vaccines could not be administered or clinics were not held at all.
The negative effect of interrupted service provision and vaccine availability on VRs and FVRs was evident in the analysis. The predominantly Datoga-affiliated sites, having the lowest VRs and FVRs, were affected by higher levels of interruption of service provision. Poor or absent service provision has been shown to be detrimental to the success of health services such as vaccinations11,45, antenatal and obstetric care46 or hospital services47, as people lose trust in the quality of the services44,46-49. There are also the opportunity costs of lost income or time away from caring for other family members which may be a disincentive for health service utilisation50-52.
The study also highlighted the negative impact of even short periods of disruption to the vaccine supply on vaccination rates45. Disruptions to vaccine supply did not affect single vaccine types equally, with some vaccines, such as oral polio vaccine, being more prone to disruptions in supply.
It is debatable whether lack of vaccines is the only explanation for the statistical difference found for BCG and measles VRs. For measles, the problems with correct registration of the vaccination in the RCH records was another likely explanation. The BCG vaccine can be given later during the first year of life, so short periods of interruption in the supply of vaccines, as documented in this study, do not explain the differences fully. The same argument applies to measles vaccination. Less than 15 days of lack of vaccine is too short an interruption to service to explain the drop in VR in 2006 and 2007. Measles vaccine could have been administered at the next clinic date. But given that interruptions in vaccine supply or scheduled clinic dates can lead to a significant loss of trust in the health system, mothers would be less likely to bring their children back for the final vaccine at another date11,44,47-49. As noted before, other studies reported that the final vaccines of an immunisation program were especially prone to high attrition rates39,40.
In multivariate analysis predominant tribal affiliation was a strong underlying effect modifier of VR and FVR. The effect of deliveries with skilled attendance was more variable. The effect of interruption of service provision persisted throughout most of the years. The effect of lack of vaccine supply on vaccination rates for the single vaccines remained almost unchanged in multivariate analysis, demonstrating its importance for vaccination coverage. Other reasons for the low VR and FVR such as educational level45,53,54, socioeconomic status39,45,54 and acceptance of vaccination services44,45,54, could not be analysed as there was no information available due to the retrospective nature of the study and the data sources.
Possible solutions to improve immunisation rates of nomadic and other difficult-to-reach populations can be found from the experience of countries such as Ethiopia, Bangladesh and Chad. In Ethiopia, local community health workers followed the pastoralist Afar tribe to locate their children in the respective locations and vaccinate them. This proactive approach made it possible to increase VRs for DPT3 from almost zero to 42% within a short period of time55, though sustaining such services over long distances and time periods is a continuing challenge. In Bangladesh, a multifaceted approach including changes to the vaccination sessions and the introduction of community support groups led to significantly higher VRs56. In Chad, an innovative approach has been developed to increase immunisation rates11,12,57,58 . In a region where almost no children had been vaccinated among nomadic pastoralists, the EPI services were combined with veterinary immunisation sessions. The importance which the pastoralist tribes placed on survival of their livestock was used to approach their infants at the same time. This enabled the health system to increase VRs for the first time above 15% in this population11,12,57,58. Cost-effectiveness studies have also demonstrated additional benefits11.
Another area needing attention relates to the service provider. Interruption of monthly service provision is detrimental to the overall success of vaccination programs, even though the actual interruption may be quite short. Similarly, interruptions to the vaccine supply will have equally detrimental effects45. Beyond these, differences in access due to mobility of populations, quality of care, and trust and confidence in providers may have accounted for the differences in coverage across the clinics.
Based on recent systematic analyses of failures within immunisation programs45,54, several areas were identified for action: immunisation services should be brought closer to the communities, involving community-based health workers and community volunteers; the program itself needs better management; actual immunisation sessions need to be aligned closer to the needs of the communities; and information for and communication with the communities (including usage of new media) must be improved59,60. Such changes can be expected to lead to significant additional improvements in immunisation coverage59,60.
The financial and structural support of immunisation programs, as provided by the Global Alliance for Vaccines and Immunization (GAVI), has improved service provision and vaccine supply to a great extent and has boosted vaccination rates considerably61. But with 19.3 million children worldwide who had not been vaccinated in 20103, the international health community should not underestimate the effort still needed to sustain and improve immunisation coverage even further59-62.
This study documented VRs and FVRs lower than the national average in a rural and partially nomadic population at different RCH clinics over several years with no consistent increase of coverage rates over time. The nomadic Datoga people seemed to have low acceptance of the RCH clinics and were less able or willing to access the immunisation clinics. These results should help inform public health care providers in restructuring these important preventive services in order to increase vaccination coverage. Equally, improved monitoring systems, improvements in vaccine supply, service delivery, and re-organisation of outreach services for nomadic communities are urgently required to increase VRs in these remote rural areas of Tanzania.
Dedication
This work is dedicated to the children and their families in the Haydom area; and to all staff at the hospital and at the RCH clinics.
References
1. WHO/UNICEF. GIVS Global immunization vision and strategy 2006-2015. Geneva: WHO, 2005.
2. Duclos P, Okwo-Bele JM, Gacic-Dobo M, Cherian T. Global immunization: status, progress, challenges and future. BMC International Health & Human Rights 2009; 9(Suppl1): S2.
3. WHO. Progress towards global immunization goals - 2010. (Online) 2010. Available: http://www.who.int/immunization_monitoring/data/SlidesGlobalImmunization.pdf (Accessed 1 October 2012).
4. UNICEF. The state of the world's children 2000. New York: UNICEF, 1999.
5. UNICEF. The state of the world's children 2009. New York: UNICEF, 2008.
6. National Bureau of Statistics (Tanzania) and Macro International Inc. Tanzania demographic and health survey 1996. Calverton, Maryland: National Bureau of Statistics (Tanzania) and Macro International Inc., 1997.
7. National Bureau of Statistics (Tanzania) and Macro International Inc. Tanzania reproductive and child health survey 1999. Calverton, Maryland: National Bureau of Statistics (Tanzania) and Macro International Inc., 2000.
8. National Bureau of Statistics (Tanzania) and ORC Macro. Tanzania demographic and health survey 2004-05. Daressalaam, Tanzania: National Bureau of Statistics and ORC Macro, 2005.
9. WHO. Measles immunization coverage among one-year-olds. (Online) Available: http://apps.who.int/whosis/data (Accessed 1 October 2012).
10. National Bureau of Statistics. Tanzania population and housing census 2002. Regional and district projections. Dar es salaam, Tanzania: National Bureau of Statistics, 2006.
11. Schelling E, Bechir M, Ahmed MA, Wyss K, Randolph TF, Zinsstag J. Human and animal vaccination delivery to remote nomadic families, Chad. Emerging Infectious Diseases 2007; 13(3): 373-379.
12. Zinsstag J, Ould Taleb M, Craig PS. Health of nomadic pastoralists: new approaches towards equity effectiveness. Editorial. Tropical Medicine & International Health 2006; 11(5): 565-568.
13. Blystad A. The pastoral Barabaig: fertility, recycling and the social order. Dissertation thesis for the Cand. Polit. degree in Social Anthropology. Bergen: Department of Social Anthropology, Faculty of Social Sciences, University of Bergen, 1992.
14. Blystad A. Precarious procreation. Datoga pastoralists at the late 20th century. Doctoral thesis. Bergen: Department of Social Anthropology, Faculty of Social Sciences, University of Bergen, 2000.
15. Hinderaker SG. Perinatal mortality and anaemia in pregnancy in rural northern Tanzania. Doctoral thesis. Bergen: Centre for International Health, Faculty of Medicine, University of Bergen, 2003.
16. Olsen BE. Motherhood - a hazardous endeavour. Maternal deaths and urinary tract infections in pregnancy in rural northern Tanzania. Doctoral thesis. Bergen: Centre for International Health, Faculty of Medicine, University of Bergen, 2002.
17. Evjen-Olsen B, Olsen OE, Kvåle G. Achieving progress in maternal and neonatal health through integrated and comprehensive healthcare services - experiences from a programme in northern Tanzania. International Journal for Equity in Health 8: 27. (Online) 2009. Available: http://www.equityhealthj.com/content/8/1/27 (Accessed 25 September 2013).
18. Flessa S. The costs of hospital services: a case study of Evangelical Lutheran Church hospitals in Tanzania. Health Policy & Planning 1998; 13(4): 397-407.
19. Maestad O, Brehony E. Review of Haydom Lutheran Hospital. CMI Report R2007:18. Bergen: Chr. Michelsen Institute, 2007.
20. Haydom Lutheran Hospital. Haydom Lutheran Hospital - annual report 1998. Haydom, Tanzania: Haydom Lutheran Hospital, 1999.
21. Haydom Lutheran Hospital. Haydom Lutheran Hospital - annual report 1999. Haydom, Tanzania: Haydom Lutheran Hospital, 2000.
22. Haydom Lutheran Hospital. Haydom Lutheran Hospital - annual report 2000. Haydom, Tanzania: Haydom Lutheran Hospital, 2001.
23. Haydom Lutheran Hospital. Haydom Lutheran Hospital - annual report 2001. Haydom, Tanzania: Haydom Lutheran Hospital, 2002.
24. Haydom Lutheran Hospital. Haydom Lutheran Hospital - annual report 2002. Haydom, Tanzania: Haydom Lutheran Hospital, 2003.
25. Haydom Lutheran Hospital. Haydom Lutheran Hospital - annual report 2003. Haydom, Tanzania: Haydom Lutheran Hospital, 2004.
26. Haydom Lutheran Hospital. Haydom Lutheran Hospital - annual report 2004. Haydom, Tanzania: Haydom Lutheran Hospital, 2005.
27. Haydom Lutheran Hospital. Haydom Lutheran Hospital - annual report 2005. Haydom, Tanzania: Haydom Lutheran Hospital, 2006.
28. Haydom Lutheran Hospital. Haydom Lutheran Hospital - annual report 2006. Haydom, Tanzania: Haydom Lutheran Hospital, 2007.
29. Haydom Lutheran Hospital. Haydom Lutheran Hospital - annual report 2007. Haydom, Tanzania: Haydom Lutheran Hospital, 2008.
30. WHO/UNICEF. United Republic of Tanzania. WHO and UNICEF estimates of immunization coverage: 2010 revision. Available: http://www.childinfo.org/files/Tanzania_United_Republic_of_1997_2010.pdf (Accessed 1 October 2012).
31. Rekdal OB. Kulturell kontinuitet og sosial endring. En studie av iraqw-folket i det nordlige Tanzania [Change and continuity in Iraqw society and culture]. Master degree thesis (in Norwegian). Bergen: Department of Social Anthropology, Faculty of Social Sciences, University of Bergen, 1991.
32. Mulder MB. Demography of pastoralists: preliminary data on the Datoga of Tanzania. Human Ecology 1992; 20(4): 383-405.
33. [Authors unlisted] Languages of Tanzania. In: MP Lewis (Ed.). Ethnologue: Languages of the world. 16th ed. Dallas, Texas: SIL International, 2009. Available: http://www.ethnologue.org/show_country.asp?name=TZ (Accessed 1 October 2012).
34. Madsen A. The Hadzabe of Tanzania. Land and human rights for a hunter-gatherer community. Document no. 98. Copenhagen: International Work Group for Indigenous Affairs, 2000.
35. Mokhtar G. (Ed.) Ancient civilizations of Africa. General history of Africa. Vol. II, abridged edition. Dar es Salaam: Tanzania Publishing House, 1990.
36. Rekdal OB. The invention by tradition: creativity and change among the Iraqw of northern Tanzania. Doctoral thesis. Bergen: Department of Social Anthropology, Faculty of Social Sciences, University of Bergen, 1999.
37. Armstrong Schellenberg JR, Mrisho M, Manzi F, Shirima K, Mbuya C, Mushi AK, et al. Health and survival of young children in southern Tanzania. BMC Public Health 2008; 8: 194.
38. Ndirangu J, Bärnighausen T, Tanser F, Tint K, Newell ML. Levels of childhood vaccination coverage and the impact of maternal HIV status on child vaccination status in rural KwaZulu-Natal, South Africa. Tropical Medicine & International Health 2009; 14(11): 1383-1393.
39. Fadnes LT, Jackson D, Engebretsen IM, Zembe W, Sanders D, Sommerfelt H, et al. Vaccination coverage and timeliness in three South African areas: a prospective study. BMC Public Health 2011; 11: 404.
40. Fadnes LT, Nankabirwa V, Sommerfelt H, Tylleskär T, Tumwine JK, Engebretsen IM, et al. Is vaccination coverage a good indicator of age-appropriate vaccination? A prospective study from Uganda. Vaccine 2011; 29(19): 3564-3570.
41. Uddin MJ, Koehlmoos TP, Saha NC, Khan IA, Shamsuzzaman. Child immunization coverage in rural hard-to-reach areas of Bangladesh. Vaccine 2010; 28(5): 1221-1225.
42. Lim SS, Stein DB, Charrow A, Murray CJ. Tracking progress towards universal childhood immunisation and the impact of global initiatives: a systematic analysis of three-dose diphtheria, tetanus, and pertussis immunisation coverage. Lancet 2008; 372(9655): 2031-2046.
43. WHO. Supplementary immunization activities calendar. Available: http://apps.who.int/immunization_monitoring/en/globalsummary/siacalendar/padvancedsia.cfm (Accessed 1 October 2012).
44. Young AG. Vaccination, harm reduction, and vulnerability among children in a northern Datoga community. Washington D.C.: Abstracts of the 2007 American Anthropology Association Annual Meeting, 28 November - 2 December 2007.
45. Rainey JJ, Watkins M, Ryman TK, Sandhu P, Bo A, Banerjee K. Reasons related to non-vaccination and under-vaccination of children in low and middle income countries: findings from a systematic review of the published literature, 1999-2009. Vaccine 2011; 29(46): 8215-8221.
46. Gabrysch S, Campbell OM. Still too far to walk: literature review of the determinants of delivery service use. BMC Pregnancy & Childbirth 2009; 9: 34.
47. Tibandebage P, Mackintosh M. The market shaping of charges, trust and abuse: health care transactions in Tanzania. Social Science & Medicine 2005; 61(7): 1385-1395.
48. Gilson L. Building trust and value in health systems in low- and middle-income countries. Editorial. Social Science & Medicine 2005; 61(7): 1381-1384.
49. Gilson L. Trust and the development of health care as a social institution. Social Science & Medicine 2003; 56(7): 1453-1468.
50. Sauerborn R, Nougtara A, Hien M, Diesfeld HJ. Seasonal variations of household costs of illness in Burkina Faso. Social Science & Medicine 1996; 43(3): 281-290.
51. Pokhrel S, Sauerborn R. Household decision-making on child health care in developing countries: the case of Nepal. Health Policy & Planning 2004; 19(4): 218-233.
52. McIntyre D, Thiede M, Dahlgren G, Whitehead M. What are the economic consequences for households of illness and of paying for health care in low- and middle-income country contexts? Social Science & Medicine 2006; 62(4): 858-865.
53. Nankabirwa V, Tylleskär T, Tumwine JK, Sommerfelt H; Promise-ebf Study Group. Maternal education is associated with vaccination status of infants less than 6 months in Eastern Uganda: a cohort study. BMC Pediatrics 2010; 10: 92.
54. Wiysonge CS, Uthman OA, Ndumbe PM, Hussey GD. Individual and contextual factors associated with low childhood immunisation coverage in sub-Saharan Africa: a multilevel analysis. PLoS One 2012; 7(5): e37905.
55. Afar Pastoralist Development Association. Mobile primary health. (Online). Available: http://www.apdaethiopia.org/MPH.htmlhttp://www.apdaethiopia.org/index.php?option=com_content&view=article&id=13&Itemid=12 (26 September 2013).
56. Uddin MJ, Saha NC, Islam Z, Khan IA, Shamsuzzaman, Quaiyum MA, et al. Improving low coverage of child immunization in rural hard-to-reach areas of Bangladesh: findings from a project using multiple interventions. Vaccine 2012; 30(2): 168-179.
57. Zinsstag J, Schelling E, Wyss K, Mahamat MB. Potentia, l of cooperation between human and animal health to strengthen health systems. Lancet 2005; 366(9503): 2142-2145.
58. Schelling E, Wyss K, Béchir M, Moto DD, Zinsstag J. Synergy between public health and veterinary services to deliver human and animal health interventions in rural low income settings. BMJ 2005; 331(7572): 1264-7.
59. Ryman TK, Dietz V, Cairns KL. Too little but not too late: results of a literature review to improve routine immunization programs in developing countries. BMC Health Services Research 2008; 8: 134.
60. Batt K, Fox-Rushby JA, Castillo-Riquelme M. The costs, effects and cost-effectiveness of strategies to increase coverage of routine immunizations in low- and middle-income countries: systematic review of the grey literature. Bulletin of the World Health Organization 2004; 82(9): 689-696.
61. Lu C, Michaud CM, Gakidou E, Khan K, Murray CJ. Effect of the Global Alliance for Vaccines and Immunisation on diphtheria, tetanus, and pertussis vaccine coverage: an independent assessment. Lancet 2006; 368(9541): 1088-1095.
62. Berhane Y, Clements CJ, Ndiaye JM, Taylor P. Has routine immunisation in Africa become endangered? Lancet Infectious Diseases 2009; 9, , (11): , 655-, 656.

